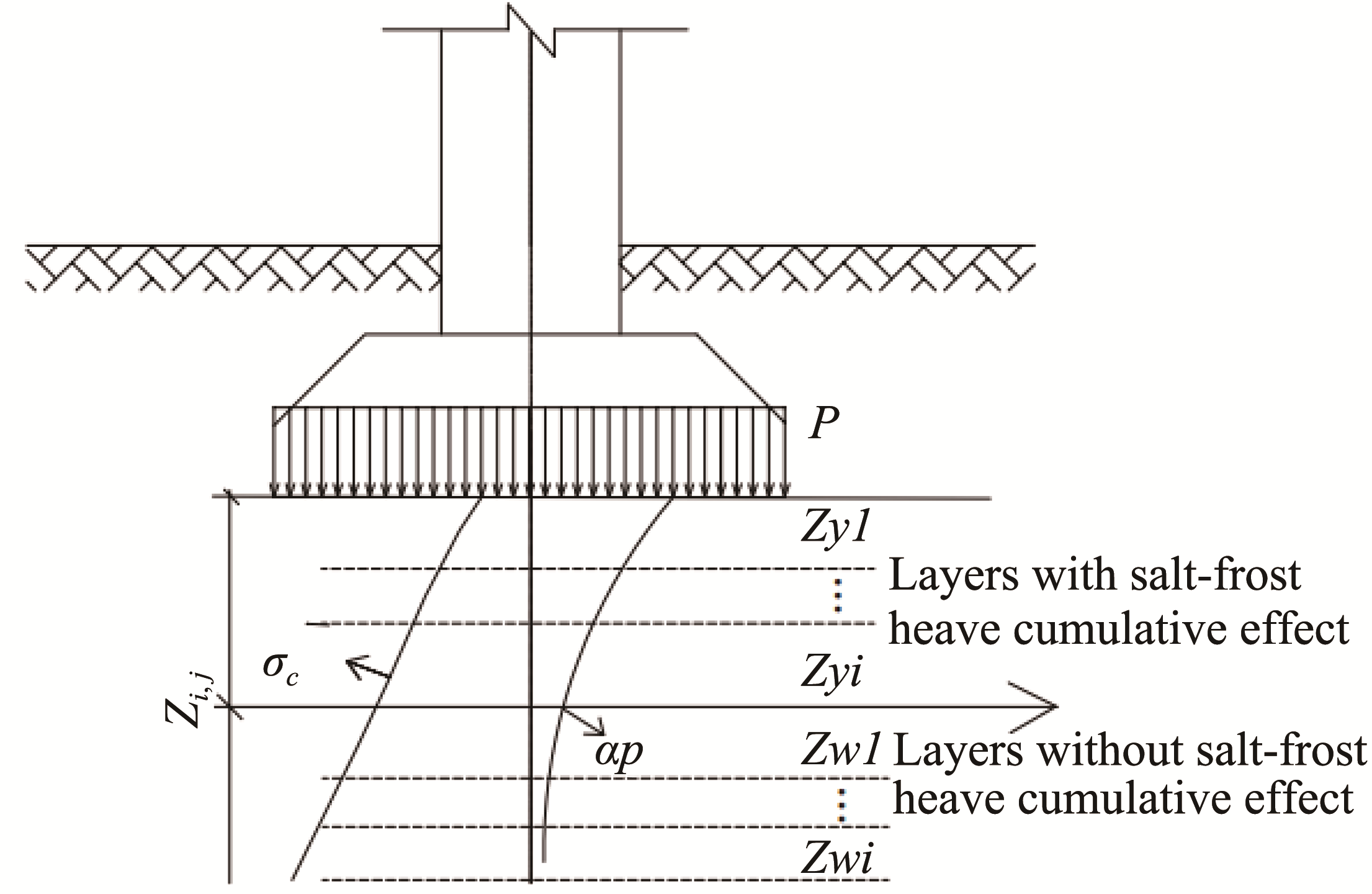
Sciences in Cold and Arid Regions ›› 2020, Vol. 12 ›› Issue (5): 284–294.doi: 10.3724/SP.J.1226.2020.00284.
• • 上一篇
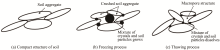
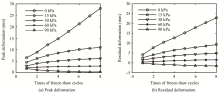
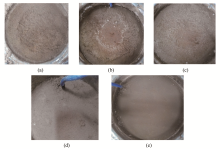
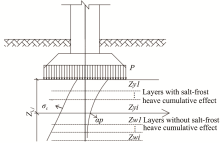
Calculation of salt-frost heave of sulfate saline soil due to long-term freeze-thaw cycles
Tao Wen1,2,Sai Ying1,3( ),FengXi Zhou3
),FengXi Zhou3
- 1.Chongqing Engineering Research Center of Building Health Monitoring and Disaster Prevention in Full-Life-Cycle, Yangtze Normal University, Chongqing, 408100, China
2.Gansu Academy of Sciences, Lanzhou, Gansu 730000, China
3.School of Civil Engineering, Lanzhou University of Technology, Lanzhou, Gansu 730050, China
|
Cheng WT, Wang Y, Wang MN, et al., 2007. Testing study on influence of freezing and thawing circulation on saline soil's cohesion. Rock and Soil Mechanics, 28(11): 2343-2347. DOI: 10.1051/0004-6361/201423882.
doi: 10.1051/0004-6361/201423882 |
|
|
Chu CP, Li B, Hou ZJ, 1998. Salt expansion accumulation of sulphate salty soil under freezing and thawing cycles. Journal of Glaciology and Geocryology, 20(2): 108-111. DOI: CNKI:SUN:BCDT.0.1998-02-002. (in Chinese)
doi: CNKI:SUN:BCDT.0.1998-02-002 |
|
|
Ding ZM, Zhang SS, Yang XH, 2008. Experimental studies of the applicability index of coarse-grained salty soil as an embankment filling. Journal of Glaciology and Geocryology, 30(4): 623-631. DOI: CNKI:SUN:BCDT.0.2008-04-015. (in Chinese)
doi: CNKI:SUN:BCDT.0.2008-04-015 |
|
|
Espinosa RM, Franke L, Deckelmann G, 2008. Model for the mechanical stress due to the salt crystallization in porous materials. Construction and Building Materials, 22: 1350-1367. DOI: 10.1016/j.conbuildmat.2007.04.013.
doi: 10.1016/j.conbuildmat.2007.04.013 |
|
|
Espinosa RM, Franke L, Deckelmann G, 2008. Phase changes of salts in porous materials: crystallization, hydration and deliquescence. Construction and Building Materials, 22: 1758-1773. DOI: 10.1016/j.conbuildmat.2007.05.005.
doi: 10.1016/j.conbuildmat.2007.05.005 |
|
|
Koniorczyk M, 2010. Modelling the phase change of salt dissolved in pore water-equilibrium and non-equilibrium approach. Construction and Building Materials, 24(7): 1119-1128. DOI: 10.1016/j.conbuildmat.2009.12.031.
doi: 10.1016/j.conbuildmat.2009.12.031 |
|
|
Koniorczyk M, Gawin D, 2012. Modelling of salt crystallization in building materials with microstructure-poromechanical approach. Construction and Building Materials, 36(4): 860-873. DOI: 10.1016/j.conbuildmat.2012.06.035.
doi: 10.1016/j.conbuildmat.2012.06.035 |
|
|
Koniorczyk M, Gawin D, Schrefler BA, 2015. Modeling evolution of frost damage in fully saturated porous materials exposed to variable hygro-thermal conditions. Computer Methods in Applied Mechanics and Engineering, 297: 38-61. DOI: 10.1016/j.cma.2015.08.015.
doi: 10.1016/j.cma.2015.08.015 |
|
|
Koniorczyk M, Gawin D, Schrefler BA, 2018. Multiphysics model for spalling prediction of brick due to in-pore salt crystallization. Computers and Structures, 196: 233-245. DOI: 10.1016/j.compstruc.2017.10.013.
doi: 10.1016/j.compstruc.2017.10.013 |
|
|
Li XX, Wang SJ, Xiao RH, et al., 2016. Saline expansion and frost heave of sodium sulfate solution during cooling crystallization process. Chinese Journal of Geotechnical Engineering, 38(11): 2069-2077. DOI: 10.11779/CJGE201611017.
doi: 10.11779/CJGE201611017 |
|
|
Niu XR, Gao JP, 2008. Deduction of salt expansion expression during pure salt expansion period of sulphate saline soil. Chinese Journal of Geotechnical Engineering, 30(7): 1058-1061. DOI: 10.3901/JME.2008.10.294.
doi: 10.3901/JME.2008.10.294 |
|
|
Niu XR, Gao JP, 2015. Expression for volume change of sulphate saline soil considering salt expansion and frost heave. Chinese Journal of Geotechnical Engineering, 37(4): 755-760. DOI: 10.11779/CJGE201504023.
doi: 10.11779/CJGE201504023 |
|
|
Scherer GW, 1999. Crystallization in pores. Cement & Concrete Research, 29: 1347-1358. DOI: 10.1016/S0008-8846(99)00002-2.
doi: 10.1016/S0008-8846(99)00002-2 |
|
| Shi ZX, Li B, Jin YC, 1994. An experimental analysis of the expanding regularity and affecting elements on sulphate salty soil. Journal of Xi'an Highway Transportation University, 14(2): 15-21. (in Chinese) | |
|
Song QZ, Chen LZ, 2006. Application of artificial neural network to studying salt expansion properties of saline soil. Journal of Glaciology and Geocryology, 28(4): 607-612. DOI: 10.1016/S1001-8042(06)60011-0.
doi: 10.1016/S1001-8042(06)60011-0 |
|
|
Steiger M, 2005. Crystal growth in porous materials—I: the crystallization pressure of large crystals. Journal of Crystal Growth, 282: 455-469. DOI: 10.1016/j.jcrysgro.2005. 05.007.
doi: 10.1016/j.jcrysgro.2005. 05.007 |
|
|
Steiger M, 2005. Crystal growth in porous materials—II: Influence of crystal size on the crystallization pressure. Journal of Crystal Growth, 282: 470-481. DOI: 10.1016/j.jcrysgro.2005.05.008.
doi: 10.1016/j.jcrysgro.2005.05.008 |
|
|
Wan XS, Hu QJ, Liao MK, 2017. Salt crystallization in cold sulfate saline soil. Cold Regions Science and Technology, 137: 36-47. DOI: 10.1016/j.coldregions.2017.02.007.
doi: 10.1016/j.coldregions.2017.02.007 |
|
|
Wang XS, Zhang HQ, Xue M, et al., 2003. Road disease and treatment in saline soil area. Journal of Tongji University, 31(10): 1178-1182. DOI: 10.3321/j.issn:0253-374X.2003.10.009.
doi: 10.3321/j.issn:0253-374X.2003.10.009 |
|
|
Wu AH, Cai LC, Gu QK, 2010. Research on ground treatment of airport with sulfate saline soil by heavy cover replacement technique. Rock and Soil Mechanics, 31(12): 3880-3886. DOI: 10.3969/j.issn.1000-7598.2010.12.030.
doi: 10.3969/j.issn.1000-7598.2010.12.030 |
|
|
Xiao ZA, Lai YM, You ZM, 2017. Experimental study on impact of salt content on deformation characteristics of sodium sulfate soil under freeze-thaw conditions. Chinese Journal of Geotechnical Engineering, 39(5): 953-960. DOI: 10.11779/CJGE201705021.
doi: 10.11779/CJGE201705021 |
|
|
Yue HM, Hang JM, Wen T, et al., 2016. Indoor simulation experiment on heavy cover replacement technique intreatment of coarse sulphate saline soil foundation. Rock and Soil Mechanics, 38(2): 471-486. DOI: 10.16285/j.rsm. 2017.02.021.
doi: 10.16285/j.rsm. 2017.02.021 |
| No related articles found! |
|
||