2. Department of Botany and Plant Biotechnology, University of Johannesburg, P. O. Box 524, Auckland Park, South Africa 2006;
3. Department of Botany, University of Lagos, Akoka, Nigeria 101017
Photosynthesis evolved early in the history of life (Blackenship, 2010). It is the process by which the chloroplast thylakoids of the leaf and other photosynthetic structures harvest light. The resultant chemical energy adenosine triphosphate and nicotinamide adenine dinucleotide phosphate (ATP and NADPH) are used to fix atmospheric carbon dioxide CO2. In C3 photosynthesis, CO2 is fixed directly via ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco). In C4 photosynthesis, CO2 is fixed indirectly after primary fixation by phosphoenol pyruvate (PEP) carboxylase and subsequent re-release in adjacent cells not in direct communication with the atmosphere, where CO2 is concentrated (Furbank, 2009). For the energy of sunlight to be stored, it must be absorbed by the pigments of the organism. Several types of pigments—such as chlorophylls, carotenoids, and phycobilins-serve this function in various photosynthetic organisms. A portion of the light energy absorbed by the pigments is eventually stored in chemical bonds. This energy conversion is a complex process involving interactions between several pigment molecules and electron-transport proteins. Collectively, these components are called a photosynthetic unit. Serving as antennae, most of the pigments collect light and channel the energy to the reaction center, where the chemical reactions leading to long-term energy storage take place (Blankenship, 1992).
Over time, photosynthetic research has enhanced our understanding of ecological phenomena and the global environment (Monsi and Saeki, 2005). Indeed, photosynthesis is now an integral component of simulation models that are used to predict the future of our planet. For a long time, improving the efficiency of photosynthesis by artificial modification of photosynthetic proteins and pathways has been considered impossible or unrealistic. Over evolutionary time, photosynthesis has become complex and tightly regulated. However, recent advances have made it possible to manipulate the process using molecular genetic engineering (Andrews and Whiney, 2003; Raines, 2006). The advancement in molecular engineering can undoubtedly have positive influences on crop productivity and yield (Parry et al., 2007 ) because photosynthetic rate depends on biomass accretion (Kruger and Volin, 2006).
To reawaken the interest of researchers in this line of study, we conducted a review of photosynthesis research in Nigeria, with a view for helping to utilize past work and to project future work for tackling new challenges and the modern application of photosynthesis in the areas of food productivity and climate change. This review is also intended to serve as a guide for possible research on C3 and C4 plants in Nigeria. Information used in this review was sourced from published research articles and books retrieved from scientific data published in reputable journals.
1.2 C3 photosynthesisThe C3 photosynthetic carbon-reduction cycle was first elucidated by Calvin, Bassham, and Benson in the 1950s in a series of experiments using the green alga Chlorella and radio-labelled carbon. This cycle, frequently referred to as the Calvin cycle, uses the products of the light reactions of photosynthesis, ATP and NADPH, to fix atmospheric CO2 into carbon skeletons that are used directly for starch and sucrose biosynthesis (Figure 1).
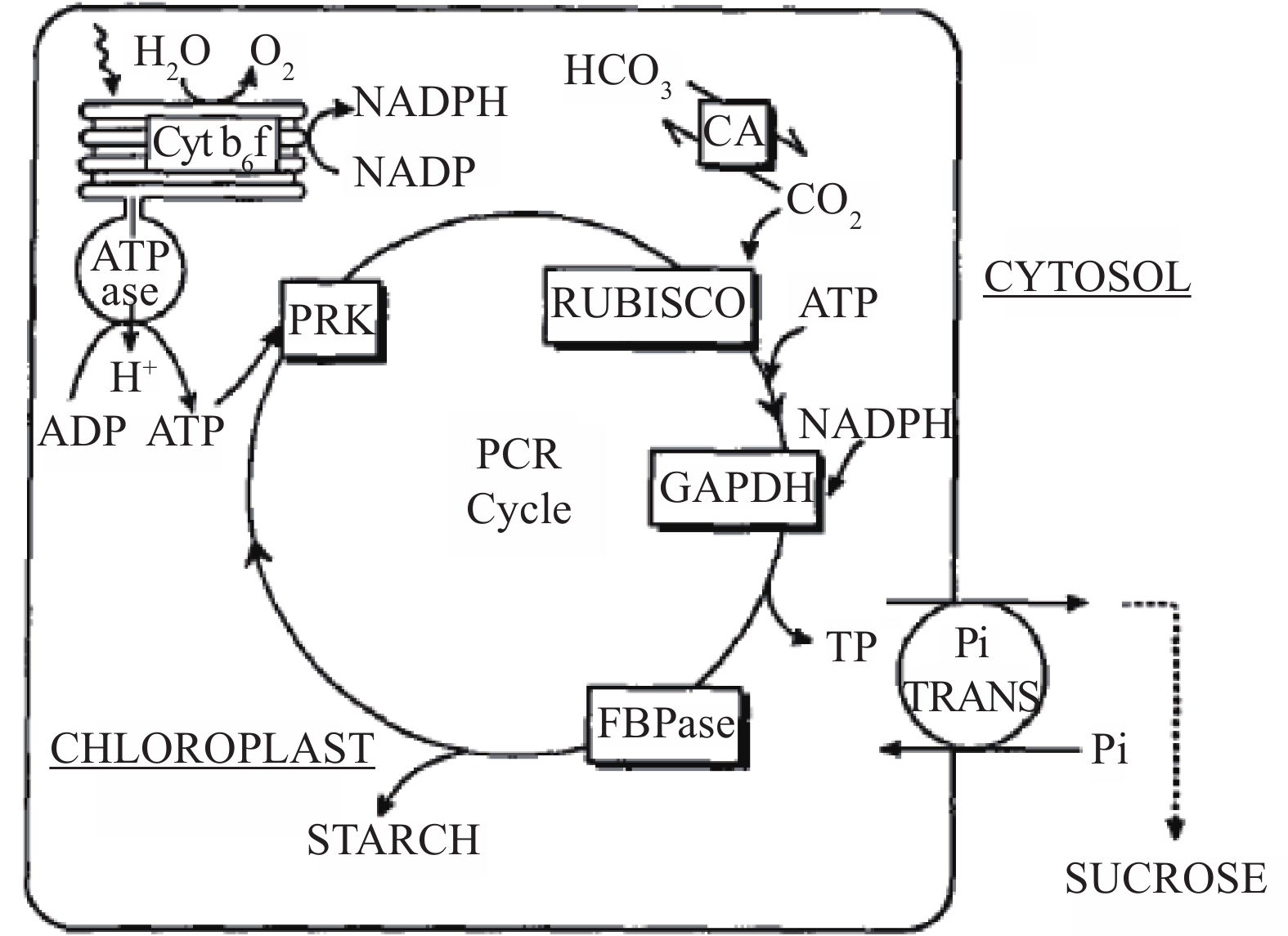
|
Figure 1 C3 pathway (photosynthetic carbon reduction, or PCR, cycle). Carbon initially fixed by RUBISCO is phosphorylated and reduced by the products of the light reactions (ATP and NADPH). The reduced three-carbon sugar-phosphate (triose phosphate, TP) can be either exported from the chloroplast for sucrose synthesis via the chloroplast envelope Pi transporter (Pi TRANS) or retained for starch synthesis or recycling to ribulose bisphosphate, the CO2 acceptor for the Rubisco enzyme. (Source: Furbank and Taylor, 1995) |
The C3 photosynthetic pathway is found in plants under moist or moderate environmental conditions (Winter et al., 1976 ). In the pathway, CO2 is fixed by Rubisco and is synthesized into carbohydrates. This pathway operates only in the mesophyll cells (Boom, 2004), and the majority of plants use this photosynthetic pathway. Most photosynthetic organisms utilize this method of carbon fixation. Except for a few taxa, all trees and shrubs and most herbs are C3 plants (Boom, 2004). These plants fix CO2 using the enzyme Rubisco and convert it in the form of a three-carbon-atom molecule storage product, 3-phosphoglyceric acid (PGA), hence the name C3 photosynthesis (Boom, 2004). In the long course of earth's history, there have been periods when conditions for C3 plants were less favourable (Berner and Canfield, 1989). Although it is generally assumed that C3 is the oldest photosynthetic pathway among higher plants, it is still surprising that far back in the evolutionary history of these plants there had been conditions that are considered unfavorable for the plants (Boom, 2004). This circumstances is because high O2 concentrations cause photorespiration in this group of plants, which causes a considerable loss of energy for the plant (Boom, 2004).
Molecularly, the C3 expression of photosynthetic carbon-reduction-cycle genes in mature leaves is sensitive to environmental and metabolic signals, providing long-term mechanisms for the plant to regulate primary carbon fixation. High levels of glucose and sucrose have been shown to be associated with reduced levels of a number of Calvin cycle mRNAs, including those encoding the small subunit of Rubisco, sedoheptulose-1,7-bisphosphatase, and fructose-1, and 6-isphosphatase. This feedback mechanism, which again appears to act at the level of transcription, might be important for source–sink regulation in the plant (Krapp et al., 1993 ). The signaling pathway involved in glucose repression of gene expression is not known although it has been suggested that the enzyme hexokinase might be involved. Recently, it has been shown that photosynthetic genes can respond at the transcript level to nutrient status (namely nitrogen and phosphorous levels) and this response is modulated by carbohydrate status. This process suggests that the interaction between carbohydrate status and nutrient status may function as a long-term strategy in the control of primary carbon metabolism.
1.3 C4 photosynthesisThe C4 photosynthetic pathway is found in plants growing in warm, sunny, and dry conditions (Winter et al., 1976 ). The leaves of C4 plants display Kranz anatomy, in which vascular bundles are surrounded by an outer layer of mesophyll cells and an inner layer of bundle-sheath cells (Brown, 1999). The C4 pathway fixes CO2 initially by phosphenol pyruvate carboxylase (PEPC) (Figure 2), which is localized in mesophyll cells forming C4 acids (malate and aspartate). The C4 plants are among the most productive on the planet, and this trait is associated with high rates of photosynthesis and efficient use of water and nitrogen (Brown, 1999). In a typical C4 plant, the initial fixation of HCO3− by PEPC and the re-fixation of CO2 by Rubisco are compartmented into different cell types although segregation can occur within individual cells (Voznesenskaya et al., 2002 ).
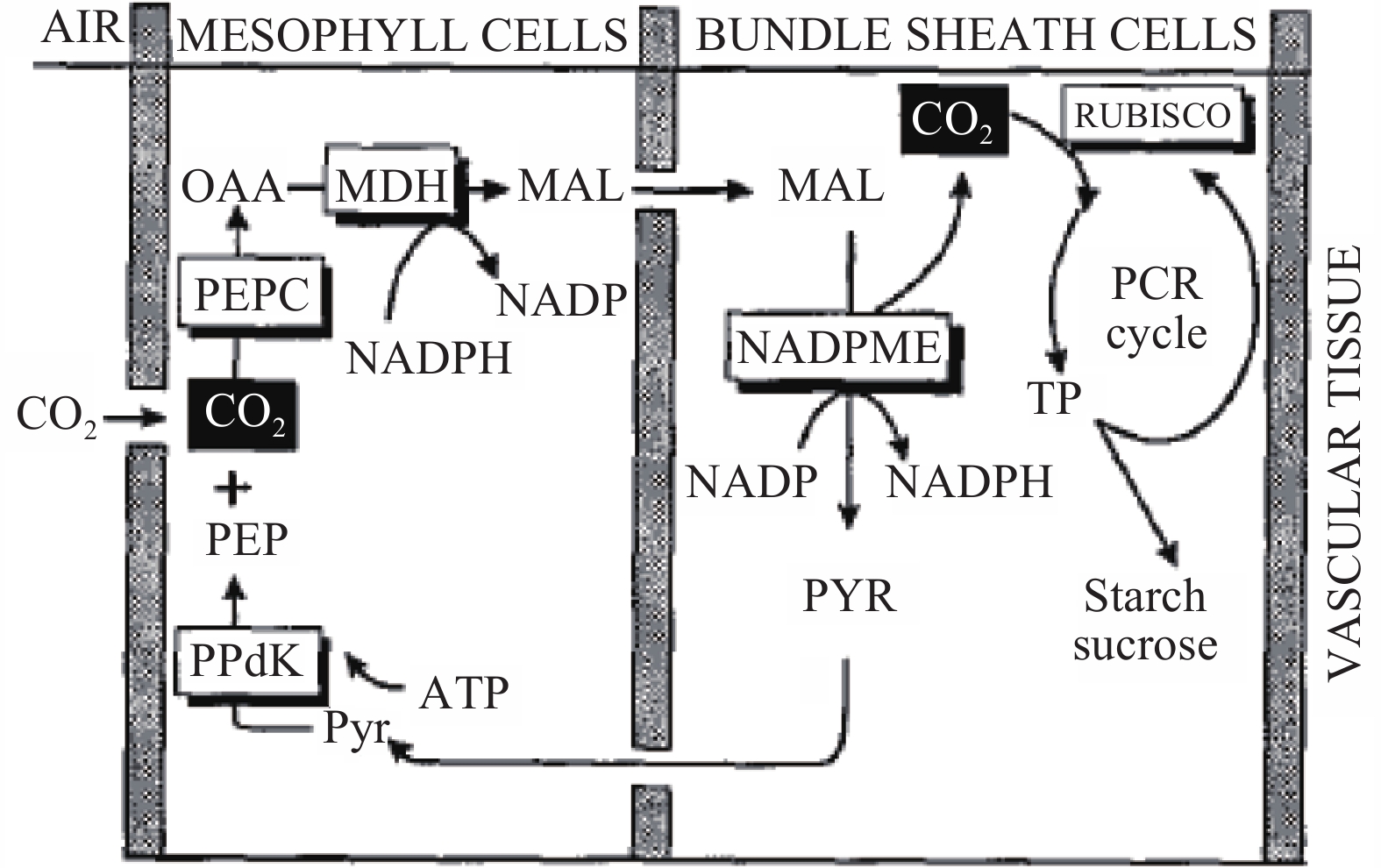
|
Figure 2 C4 pathway, simplified to describe only the NADP-malic enzyme type, which transports malate from the mesophyll to the bundle-sheath chloroplasts. CO2 is fixed by the enzyme PEPC to form the C4 acid oxaloacetate (OAA), which is reduced by NADPH from the light reactions to form malate (MAL), the C4, acid that is transported to the bundle-sheath cells. Malate is decarboxylated in the bundle-sheath cells, where the CO2 released is fixed by the PCR cycle in much the same way as in C3 plants. The three-carbon compound pyruvate (PYR) diffuses back to the mesophyll, where it is phosphorylated by ATP to regenerate the carbon acceptor. phosphoenolpyruvate (PEP) (Source: Furbank and Taylor, 1995) |
C4 photosynthesis is found in many plant species: monocots including Zea, Cyperus and Sacharrum; dicots such as Amaranth, Flaveria, Euphorbia, Cyperus, Cleome, and Boerhavia (Jørgensen and Ulloa, 1994; Ehleringer et al., 1997 ; Muhaidat et al., 2007 ). Table 1 presents some of the Nigerian plants with C3 and C4 features. Such a wide and diverse distribution of the occurrence of the C4 pathway indicates that it has evolved independently at many different times (Langdale and Nelson, 1992). Because all C4 plants share a number of characteristics in terms of leaf anatomy, physiology, and biochemistry, they stand as excellent examples of convergent evolution. The C4 pathway provides a means for maintaining photosynthetic efficiency under conditions of high temperature or water limitation, by greatly reducing or eliminating photorespiration (Ehleringer et al., 1997 ). Photorespiration is problematic in these situations because increasing temperatures enhance the oxygenase activity relative to the carboxylation activity of the Rubisco enzyme (Ehleringer et al., 1997 ). In addition, C4 plants have a selective advantage under arid conditions. To minimize water loss through transpiration, plants must reduce the opening of their stomata, which leads to reduced carbon CO2 uptake and reduced release of oxygen. Because PEPC can fix carbon dioxide from relatively low intracellular concentrations, C4 plants show higher rates of photosynthesis than C3 plants under conditions that promote high transpiration rates (Sage, 2004). C4 plants also show higher photosynthetic efficiencies under conditions of light saturation, such as occurs in open plains or Savannahs (Lattanzi, 2010). It is likely that the ancestors of most contemporary C4 and C3–C4 intermediate plants evolved between 30 and 50 million years ago, in response to reduced carbon dioxide in the atmosphere, possibly in combination with elevated temperatures or water deficit (Ehleringer et al., 1997 ).
The occurrence of C3–C4 indicates that multiple independent steps are required for evolution of full C4 capability. In most C4 plants, efficient transfer of metabolites between mesophyll and bundle-sheath cells is as a result of higher plasmodesmatal linkage between these two cells, as compared to C3 species (Botha, 1992). In the mesophyll cells of C4 plants, chloroplast development is reduced; while in bundle sheath cells, chloroplasts are larger and undergo additional rounds of division (Hatch, 1987). According to Kanai and Edwards (1999), the operation of the C4 cycle results in the increased concentration of CO2 at the active site of Rubisco in the bundle-sheath cells by suppression of the oxygenase reaction of Rubisco. C4 plants have evolved independently from C3 plant in different taxonomic groups (Björkman, 1976), and the photosynthesis is more efficient than C3 photosynthesis under some environmental conditions. C4 plants fix atmospheric CO2 through Phosphoenolpyruvate Carboxylase (PEPC) in the mesophyll cells to form oxaloacetate, which is converted to four-carbon decarboxylic acids (malate and aspartate) (Larcher, 1995). The four-carbon acids are then translocated to bundle-sheath cells and decarboxylated at the C4 position with the release of CO2 and C3 compound (Larcher, 1995). C4 plants have been reported to show higher export rates of photosynthate than C3 plants (Leonardos and Grodzinski, 2000). To explain the higher rate of translocation, some researchers have pointed to the presence of a denser vascular system in C4 leaves (Crookston and Moss, 1974) and a larger cross-sectional area of phloem (Gallaher et al., 1975 ) to be responsible for it. The vascular bundle is composed of two kinds of conducting tissues: xylem and phloem. Thus, it appears that C4 leaves have a denser hydraulic network than C3 leaves. The CO2-concentrating mechanism of the C4 pathway gives C4 plants an efficient photosynthetic mechanism under low stomatal conductance and thus a higher water-use efficiency and photosynthetic ability under environments of low water availability than is the case for C3 plants (Sage, 2004). Nevertheless, it is a generally accepted fact that C4 plants evolved from C3 plants; and the evolution was accompanied by modifications to anatomical and biochemical features of leaves. A change in the vein density of leaves undoubtedly occurred during the evolution from C3 to C4 plants (Sage, 2004; Ueno and Sentoku, 2006).
According to Hatch (1987), C4 plants are divided into three subtypes, differing in the process of decarboxylation of C4 acids; nicotinamide adenine dinucleotide phosphate-malic enzyme (NADP-ME), nicotinamide adenine dinucleotide malic enzyme (NAD-ME), and phosphoenolpyruvate carboxykinase (PCK). The difference in their biochemical function is associated with the structural features of the leaves. While the NADP-ME grasses have a bundle shealth that originated from the mestome sheath, both the NAD-ME and PCK grasses have one that originated from the parenchyma sheath (Dengler and Nelson, 1999). The bundle-sheath cells of the C4 subtypes also differ in the structure, intracellular position, and quantity of chloroplasts and mitochondria (Yoshimura et al., 2004 ). The quantitative balance of photosynthetic tissues (Dengler et al., 1994 ) and organelles (Yoshimura et al., 2004 ) between the mesophyll and bundle-shealth cells reflects the difference in biochemical function of the photosynthetic subtypes.
2 Methods used in determining photosynthetic pathways in plants 2.1 Photosynthetic productsInitial product labeling with 14C is the only direct method for photosynthetic pathway determination. Hatch et al. (1967) reported that, in C4 plants, as much as 93% of fixed radioactivity appeared in oxaloacetic, malic, and aspartic acids following exposure to 14CO2 for approximately one second. In contrast, early products of the C3 process were 3-PGA and hexose phosphates.
2.2 Carbon-dioxide compensation and photorespirationThe carbon-dioxide compensation point (the point at which photosynthetic CO2 uptake equals respiratory CO2 evolution when measured in a closed chamber) is an easily quantifiable characteristic. During photosynthesis, a light-induced release of CO2 can occur and is referred to as photorespiration, as contrasted with CO2 released by mitochondria or dark respiration. Plants with the C4 pathway have a photosynthetic CO2 compensation in the range of 0~10 ppm, indicating a lack of significant net photorespiration (Downton and Tregunna, 1968). Photorespiration occurs as a normal product of the Calvin cycle within the bundle-sheath cells of C4 plants. However, because the mesophyll layer surrounds the bundle sheath, the C4 pathway would rapidly refix any photorespiratory CO2 and prevent leakage to the atmosphere (Bowes and Ogren, 1972). A much higher CO2 compensation point (37~70 ppm) is characteristic of C3 plants (Black, 1971). Carbon-dioxide compensation points provide a convenient means of identifying the type of photosynthetic pathway. The low CO2 compensation point of C4 plants indicates an ability to utilize more external CO2, as compared to use by C3 plants.
2.3 Oxygen suppressionOxygen differentially affects CO2 exchange in C3 and C4 plant species, primarily because of differences in photorespiration. In soybean (Glycine max), and probably other C3 species as well, the total O2 inhibition consists of two discernible effects (Waller and Lewis, 1979). Oxygen substitutes for CO2 in the carboxylase reaction to yield P-glycolate, a C3 photorespiratory intermediate. As a result of this substitution, O2 competitively inhibits the carboxylase with respect to CO2 (Ogren, 1976). During photorespiration, glycolate is oxidized, releasing CO2. Consequently, oxygen depletion reduces glycolate oxidation, thereby increasing photosynthetic CO2 assimilation by 40% ~ 50% in species possessing the C3 pathway, while having no effect on C4 plants (Downes and Hesketh, 1968). The re-fixation of photorespiratory CO2 allows C4 plants to utilize all of the fixed CO2, thus increasing photosynthetic efficiency. Chollet (1976) postulated that the enzyme complement of the C4 pathway increased CO2 concentration at the site of the C3 carboxylase, reducing the competitive inhibition of O2 and minimizing photorespiration. The CO2 concentration at the site of the C3 carboxylase, coupled with a specialized leaf anatomy allowing recapture of photorespiratory CO2, was apparently responsible for the lack of photorespiration and the absence of an inhibitory effect of 21% O2 on net photosynthesis in C4 plants (Waller and Lewis, 1979).
2.4 Carbon-isotope discriminationCarbon-isotope ratio (13C/12C) in plant tissue is characteristically is known to be less than that of atmospheric CO2, indicating that plants preferentially assimilate the lighter of the two isotopes (Troughton et al., 1974 ). Carbon-isotope values are defined as the difference in per 13 mil (1 mile = 0.0254 mm) of the 13C/12C ratio of the sample relative to a standard and reported as 13C‰ (Smith and Epstein, 1971). Details of the procedure are described elsewhere (Park and Epstein, 1960). Higher plants are placed into two categories, those with low 13C‰ values (−24‰ to −34‰) and those with high values (−6‰ to −19‰) (Smith and Epstein, 1971). The distinctive difference apparently results from differences in affinity of the enzyme systems of the two pathways for the two isotopes of carbon (Whelan et al., 1973 ). Thus, the carbon-isotope technique was cited as a reliable method of distinguishing between C3 and C4 plants (Bender, 1971). This method was successfully used by Cornet and Bonhomme (2007) to characterize 23 genotype of Dioscorea; they indicated that the δ13C values for all yam samples studied ranged from −25.39‰ to −30.07‰, which indicated all species had a C 3 photosynthetic type.
2.5 Leaf anatomyLeaf anatomy provides easily distinguished differences between C3 and C4 plants. C4 photosynthesis is characterized with leaf property called "Kranz" anatomy (after Haberlandt's description in German of a wreathlike arrangement of cells). Kranz anatomy can be described as two distinct concentric layers of chlorenchyma cells formed by a bundle sheath containing most of the chloroplasts, surrounded by an outer layer consisting of a small number of mesophyll cells (Carolin et al., 1973 ). The visual identification of such arrangements in transverse section has been used in numerous anatomical surveys of leaves to identify the photosynthetic pathway for hundreds of species (Renvoize, 1987; Faniyan et al., 2013; Ayeni et al., 2015a ; Ajao et al., 2017 ). Plants with the C4 photosynthetic pathway generally have well-developed parenchymatic bundle sheaths containing high concentrations of chloroplasts and starch. Bundle-sheath cells utilize the C3 photosynthetic process; however, they are surrounded by mesophyll cells containing chloroplasts utilizing the C4 photosynthetic process, which fix and then supply CO2 for the C3 pathway.
Anatomical characteristics that have been used to distinguish plant species with both C3 and C4 photosynthetic pathways include stomatal density (Oguro et al., 1985 ; Kim, 2012; Ajao et al., 2017 ); interstomata distance (Oguro et al., 1985 ); stomatal index and stomatal size (Taylor et al., 2010 ; Ajao et al., 2017 ); Kranz tissue (Bruhl and Wilson, 2007; Martins and Alves, 2009); interveinal distance (Nelson et al., 2005 ; Soros and Dengler, 1998; Ajao et al., 2017 ); leaf thickness (Nelson et al., 2005 ; Marshall et al., 2007 ; Ajao et al., 2017 ); mesophyll thickness (Nelson et al., 2005 ); intercellular air spaces (Marshall et al., 2007 ; Ajao et al., 2017 ); one-cell criterion (Soros and Dengler, 1998; Ajao et al., 2017 ); and maximum lateral cell count (Soros and Dengler, 1998; Bruhl and Wilson, 2007; Ajao et al., 2017 ). Leaf vascular traits such as leaf-vein density and vascular spacing are also known to be diverse across C3 and C4 flowering plant species (Nelson and Dengler, 1997; Ajao et al., 2017 ) because of their importance in leaf physiological functions such as photosynthesis and water-use efficiency (Mckown et al., 2010). The most important among these anatomical features is the occurrence of Kranz anatomy.
A number of researchers have used anatomical characteristics to classify plants along C3 and C4 photosynthetic pathways. For example, Welkie and Caldwell (1970) worked on the leaf anatomy of species in some dicotyledon families, as related to the C3 and C4 pathways of carbon fixation. Also, Hattersley et al. (1982) reported on leaf anatomical variations in Neurachne and its relatives, in relation to C3 and C4 photosynthesis. Oguro et al. (1985) compared leaves of C3 and C4 species of Panicum (Poaceae), using anatomical and morphological characteristics. In addition, Dengler et al. (1994) studied the quantitative leaf anatomy of C3 and C4 species of Poaceae, considering the bundle sheath and mesophyll surface-area relationship. Another interesting report is that of Ueno et al. (2006) , which used the criteria of differentiation of some amphibious species of Eleocharis in Cyperacea. The paper reports that Eleocharis vivipara has a unique nature that expresses C4 characteristic under terrestrial conditions and C3 characteristics under submerged conditions. In a similar way, Fisher et al. (1997) subjected all North American species of the halophytic genus Suaeda (Chenopodiaceae) to C3 and C4 photosynthetic pathways classification using their leaf anatomy, which agrees with already established classification based on morphological characters. In their report, they showed that C3 species belong to the Chenopodina group, while C4 species are in the Linbogermen group. Nelson et al. (2005) studied functional leaf anatomy of some dicots in Canada, using characteristics such as leaf thickness, mesophyll thickness, and intercellular air space to compare crassulacean acid metabolism (CAM) plants with C3 and C4 plants. Soros and Dengler (1998) studied quantitative leaf anatomy of C3 and C4 Cyperaceae and comparisons with Poaceae. Huxman and Monson (2003) worked on stomatal responses of C3, C3–C4 intermediates, and C4 Flaveria species to light and intercellular CO2 concentration.
2.6 Leaf and inflorescence morphologyStudies on morphological characteristics (vegetative or floral) that could possibly be used as indicators in the classification of plant species into C3 and C4 photosynthetic groups are rare (Ayeni et al., 2015b ). Interestingly, previous reports have suggested that some morphological characteristics are related and can be linked with the two identified photosynthetic pathways in the Cyperus genus. Muasya et al. (2009) reported that in this genus, those belonging to the C3 photosynthetic pathways tend to possess spikelets arranged in digitate clusters, while the C4 counterpart spikelets are usually spicately arranged. This finding is also in line with the report of Larridon et al. (2011) , that genus Cyperus is most commonly divided into two main infrageneric units, determined by the state of a set of anatomical and inflorescence characteristics. Ayeni et al. (2015b) reported the first known work on the use of morphological characteristics to delimit Nigeria species of Cyperus into C3 and C4 pathways.
3 An overview of C3 and C4 plants research in NigeriaThe literature survey conducted on previous work such as Gentry (1993), Jorgensen and Ulloa (1994) and Pinto-Escobar and Mora-Osejo (1966) revealed that C3 and C4 plants are distributed among 21 genera and 11 families in Nigeria (Table 1).
|
|
Table 1 List of plants with C3 and C4 photosynthesis in Nigeria |
Out of the 21 genera, 3 general including Euphorbia, Cyperus and Boerhavia have been classified into C3 and C4 by Faniyan et al., 2013; Ayeni et al., 2015a , b; Ajao et al., 2016 , 2017. This thus infers that research into this line of thought is still not optimally explored, despite the fact that Nigeria is endowed with vast array of C3 and C4 plants. Although many of the species listed in the table have been classified based on stable anatomical characters such as stomata type, trichomes, and arrangement of the cell without putting into consideration the photosynthetic pathways exhibited by the plants. All the previously reported work on C3 and C4 plant in the country employed the use of anatomical character to delimit the species into their respective pathways, with pioneering one being that of Faniyan et al. (2013), where they used the occurrence of Kranz structure, one cell distant count and interveinal distance to classify four species of Euphorbia. Their study revealed that E. hirta and E. hyssopifolia are C4 while E. heterophyla and E. graminea are C3. They also found out that characters such as stomatal density, cell sizes, vein-stomatal distance, inter-stomatal distance, leaf thickness, mesophyll thickness, and intercellular air spaces were not found to be useful in their classification.
Two years later, Ayeni et al. (2015a) employed leaf anatomical characters to delimit twelve species of Cyperus into C3 and C4. The study revealed the usefulness of a combination of anatomical characters, rather than isolated ones, in the grouping of plant species according to their photosynthetic pathway. The combination of characters that was reported to be useful in their study are Kranz tissue, maximum cell distant count, maximum lateral cell count, interveinal distance, and to some extent, leaf and mesophyll thickness, were also found to be useful to classify Cyperus species into their respective photosynthetic pathways. This study confirmed the photosynthetic pathway utilized by C. difformis and C. haspan to be C3, while that of C. articulatus, C. compressus, C. distans, C. esculentus, C. imbricatus, C. iria, C. rotundus, C. sphacelatus and C. tenuiculmis is C4.
Interestingly, Ayeni et al. (2015b) also reported that floral morphological characteristics have a potential to be useful in the grouping of Cyperus species as either C3 or C4 species. They found that compound umbellate inflorescence, digitate spikelets, and spikelet length not over 1 cm are may be peculiar to C3 species, while C4 species have simple umbellate inflorescence, spicate spikelets, and spikelet length over 1 cm. The two papers demonstrated a reliable basis for assessing photosynthetic pathways of the investigated Cyperus species in Nigeria, using inflorescence morphology and anatomical characters.
Lastly, in continuation of our effort to classically delimit our native species along the photosynthetic lines, Ajao et al. (2016 , 2017), reported the first known photosynthetic delimitation of genus Boerhavia into C3 and C4 pathways. In the study, characters such as stomata index, stomata size, inter-stomatal distance, stomatal density, interveinal distance, intercellular air spaces, leaf thickness, mesophyll thickness, Kranz tissue, one cell distant count criterion, maximum lateral cell count criterion, vein density and vein distance was useful in the grouping three species B. erecta, B. coccinea and B. repens into C4 plants and B. diffusa into C3 plant. Also in the study, the characters including interveinal distance less than 166 μm and maximum lateral count ranging 2~6 were touted to be diagnostic for C4 dicotyledons species. The submission is due to the fact that higher interveinal and maximum lateral counts has been reported for monocotyledons. To the best of our knowledge, there is a dearth of information to date on the physiological and biochemical characterization of C3 and C4 plants in Nigeria. Research into this direction is imperative to complement the previous studies.

|
Figure 3 Vegetative and floral morphology of some Cyperus species studied. C. difformis (C3)—A (vegetative), B (floral); C. haspan (C3)—C (vegetative), D (floral); C. articulatus—(C4), E (vegetative), F (floral); C. dilatatus (C4), —G (vegetative), H (floral). Scale bar: A, C, E, and G = 5 cm; B, D, F, and H = 0.5 cm (Ayeni et al., 2015b ) |

|
Figure 4 Leaf transverse sections of some Cyperus species. (A) and (B), C. difformis (C3 species); (C) and (D), C. imbricatus (C4 species); and (E) and (F), C. dilatatus (C4 species). IVD: Interveinal distance, VB: vascular bundle, MS: mestome sheath, PBS: parenchymatous bundle sheath, CC: clorenchymatous cells, KS: Kranz sheath. Scale bar: (A), (C), and (E): 85 mm; (B), (D), and (F): 21 mm. (Source: Ayeni et al., 2015b ) |
In other African countries such as Ghana and South Africa, research into C3 and C4 plant has reached considerable level. In Ghana, awareness has been created on the need to cultivated C4 plants in other to meet the demand for fossil fuel in the next few decades, as it is touted that there will be rise in fossil fuel demand and prices (Black et al., 2012 ). In South Africa, Stock et al. (2004) used δ13C values of herbarium specimens of 68 southern African species from 22 genera and eight tribes to delimit the species to either the C3 or C4 photosynthetic pathway. Out of the 68 species studied, 28 exhibited C4 photosynthesis distributed among nine genera of four tribes (Cypereae, Scirpeae, Abildgaardieae and Rhyncosporeae). Their study also revealed the absence of strong relationships in the abundance of C4 plant and climatic factors such as altitude and rainfall in the country. Vogel et al. (1978) investigated the distribution of C3 and C4 grasses in South Africa. They found out that C3 and C4 grasses co-occupied areas that have minimum of 100 mm of annual rainfall and maximum of 1000 mm. Furthermore, low temperatures below 25 also favors C3 grasses when compared to C4 counterparts. And, studies focusing on energy dissipation of C4 plants under water stress have been reported (Lal and Edwards 1996; Ripley et al., 2007 ). The phylogenetic issue on Alloteropsis semialata, a South African grass with C3 and C4 photosynthesis has also been resolved (Ripley et al., 2007 ).
In West Africa at large, the influence of C3 and C4 vegetation on soil organic matter dynamic in semi-natural tropical ecosystems including Sahel savanna, Sudan savanna, savanna woodland and the dry forest has been investigated (Saiz et al., 2015 ). They found out that current environmental conditions encourage the distribution of C3 plants over their C4 counterparts in more mesic savanna ecosystems of West Africa. In addition, δ13C was also found to be varied with soil across the ecosystem. In addition, the interdependence between biotic and abiotic factors undoubtedly has influence on whether soil organic matter dynamics of C3 and C4 derived vegetation will vary in ecosystems where both vegetation types coexist. In Southern Africa, nitrogen isotopic (δ15N) abundances on soils and C3 and C4 plants along land use gradients of varying aridity has been investigated (Aranibar et al., 2008 ). The δ15N values of soils and plants were found to be higher in the areas with significant land use intensity. However, their study also revealed C3 plants significantly have higher δ15N than C4 counterparts from the same area. In another study conducted by Swap et al. (2004) , the mean annual precipitation and the δ15N of the C3 vegetation of southern African were found to be inversely proportional whereas, on the contrary, no relationship was found between mean annual precipitation and the δ13C and δ15N signatures of C4 vegetation.
4 Future research and its application in food security, global climate change, and biofuel in NigeriaPhotosynthesis is one of the main research objects in many studies on the impact of water deficit on plants (Ghannoum, 2009). Water deficit is one of the worst disasters that affect health and activities in the world. The changes in precipitation patterns and the expansion of waterlogging or of drought-affected areas are expected in the future due to global climate change (Xoconstle-Cazáres et al., 2010 ). The response of C3 photosynthesis to water stress has been well studied (Flexas et al., 2004 ). In general, the literature points to the fact that C3 photosynthesis is negatively affected by water stress, measured as changes in leaf-water potential or relative water content. In contrast, the response of C4 photosynthesis to water stress has been less studied. In spite the fact that C4 plants make a significant contribution to the global carbon budget, and C4 crops, such as maize and sorghum, are pivotal to current and future global food security (Lloyd and Farquhar, 1994; Brown, 1999; Pingali, 2001). The CO2-concentrating mechanism, compared to the C3 photosynthetic pathway, ensures a higher carbon-assimilation rate and dry-matter production under drought conditions, when stomata closure reduces CO2 supply (Larcher, 1995). Consequently, higher stomatal resistance allows a low transpiration rate and high water-use efficiency in C4 plants (Zhang and Kirkham, 1995).
Breeding of drought-resistant plants can be an advantageous strategy for avoiding drought-induced damage to agriculture. A suitable alternative to ordinary crops is C4 plants, such as maize, sorghum, millet, and amaranth. These plants are characterized by high productivity (Svirskis, 2009), nutritive value, and resistance to water deficit (Liu and Stutzel, 2004; Osborne and Freckleton, 2009). They dominate in arid and hot regions; approximately half of the world's grasses and only from 4%~10% of all plant species use the C4 photosynthetic pathway. Nevertheless, high photosynthetic capacity and productivity of these plants determine their essential contribution to global primary production (Osborne and Freckleton, 2009).
As the world population races toward 10 billion, agricultural scientists are realizing that another "green revolution" is needed for crop yields to meet demands for food. In rice, yield potential is limited by the photosynthetic capacity of leaves that, as carbohydrate factories, are unable to fill the larger number of florets of modern rice plants. One potential solution is to introduce a higher-capacity photosynthetic mechanism like the C4 pathway into rice. This is the goal of researchers in the international C4. Rice Consortium: to identify and engineer the genes necessary to install C4 photosynthesis in rice (Hibberd et al., 2008 ). Rubisco, the primary CO2-fixing enzyme in rice, is a poor catalyst of CO2 under present-day atmospheric conditions. It has a tendency of confusing its substrate CO2 with the more abundant O2, as well as being a very slow catalyst of CO2, turning over only once or twice per second. Rubisco's oxygenase activity requires the re-cycling of phosphoglycolate in the photorespiratory pathway, resulting in an energy cost and loss of previously fixed CO2. Many plants have developed active CO2-concentrating mechanisms to overcome Rubisco's inefficiencies among land plants; and this has led to the development of C4 photosynthesis, a biochemical CO2-concentrating mechanism. C4 photosynthesis arose multiple times in the past 60 million years in warm, semi-arid regions, with early occurrences coinciding with low atmospheric CO2 in the late Oligocene (Sage et al., 2011 ).
A C4 rice is achievable with a subset of C4 genes, but it will require substantial fine-tuning of biochemistry and anatomy. Particularly intriguing is the need for additional metabolite transport across membranes of organelles in C4 photosynthesis (Langdale, 2011). A functional C4-concentrating mechanism in rice would allow for an approximately two-thirds reduction in Rubisco levels, relative to wild-type rice; but Rubisco would be sequestered in bundle-sheath cells and ideally have a greater catalytic turnover rate (Badger et al., 1998 ). However, it is expected that another few years of research is required for optimization of the phenotype and field-testing for C4 rice to become ready for cultivation in farmers' fields.
Another driver of the current C4 research agenda is the global focus on biofuels. Two of the current major biofuel crops, sugarcane and maize, are both C4 species. Whereas the future of sugar cane as a fuel crop is almost certain, the use of maize can be defended only in a future in which lignocellulosic fermentation means that grain is not used to produce ethanol. However, another C4 species may hold the key to biofuel demands, for example, in the United States of America. The perennial grass Miscanthus giganteus is capable of producing a higher biomass than maize, primarily because it can photosynthesize efficiently for a longer period during the growing season. This increased efficiency is achieved in two ways. First, Miscanthus can photosynthesize at cooler temperatures than maize, as a consequence of cold-tolerant pyruvate phosphate dikinase (PPdK) activity (Wang et al., 2008 ). Second, its perennial habit makes it possible for the plant to capture more light early in the season because at that time the canopy is bigger than that of annual crops such as maize (Dohleman and Long, 2009). Current estimates suggest that 9.7 million hectares (1 hectare = 10,000 m2) of Miscanthus would provide enough biomass to meet the annual U.S. energy mandate (Somerville et al., 2010 ). Given that long-term field trials have shown that Miscanthus produces high yields even on poor soils and that 14 million hectares (1 hectare = 10,000 m2) of land dropped out of agricultural use in the U.S. between 1997 and 2007 (http://www.ers.usda.gov/statefacts/us.htm), this C4 perennial could resolve the food versus fuel dilemma in the U.S. for the foreseeable future.
This trending C4 research has resulted in one of the largest consortia of plant biologists pursuing a common goal. Nigeria can also optimistically take on this challenge, anticipating that advances in our understanding of C3 and C4 photosynthesis will better serve humanity in years to come by rejuvenating our economy in the areas of food production and green energy.
Acknowledgments:The authors thank Dr. Sabiu Saheed for encouraging us to write this review; also, the support of Mr. Alayande Kazeem and Dr. Balogun Fatai Oladunni is greatly appreciated.
Ajao AA, Jimoh MA, Saheed SA. 2017. Studies on anatomical characters indicating C3 and C4 photosynthetic metabolism in the genus Boerhavia L. (Nyctaginaceae)
. Taiwania, 62(3): 265-271. DOI:10.6165/tai.2017.62.265 |
Ajao AA, Jimoh MA, Saheed SA. 2016. Studies on anatomical characters indicating C3 and C4 photosynthetic metabolism in the genus Boerhavia L. in Nigeria
. South African Journal of Botany, 103: 305-306. DOI:10.1016/J.SAJB.2016.02.011 |
Andrews TJ, Whitney SM. 2003. Manipulating ribulose bisphosphate carboxylase/oxygenase in the chloroplasts of higher plants. Archives of Biochemistry and Biophysics, 414(2): 159-169. DOI:10.1016/S0003-9861(03)00100-0 |
Aranibara JN, Andersonb IC, Epsteina HE. 2008. Nitrogen isotope composition of soils, C3 and C4 plants along land use gradients in southern Africa
. Journal of Arid Environments, 72: 326-337. DOI:10.1016/j.jaridenv.2007.06.007 |
Ayeni OB, Jimoh MA, Saheed SA. 2015a. Leaf anatomical characters in relation to the C3 and C4 photosynthetic pathway in Cyperus (Cyperaceae)
. Nordic Journal of Botany, 33(3): 318-323. DOI:10.1111/njb.00710 |
Ayeni OB, Jimoh MA, Saheed SA. 2015b. Inflorescence and floral characters indicating C3 and C4 photosynthesis in some species of the genus Cyperus L. (Cyperaceae)
. International Journal of Biological and Chemical Sciences, 9(4): 1844-1850. DOI:10.4314/ijbcs.v9i4.10 |
Badger MR, Whitney SM, Ludwig W, et al. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae
. Canadian Journal of Botany, 76(6): 1052-1071. DOI:10.1139/b98-074 |
Bender MM. 1971. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation
. Phytochemistry, 10(6): 1239-1244. DOI:10.1016/S0031-9422(00)84324-1 |
Berner RA, Canfield DE. 1989. A new model for atmospheric oxygen over Phanerozoic time. American Journal of Science, 289(4): 333-361. DOI:10.2475/ajs.289.4.333 |
Bisalputra T, Downton WJS, Tregunna EB. 1969. The distribution and ultrastructure of chloroplasts in leaves differing in photosynthetic carbon metabolism. I. Wheat, Sorghum, and Aristida (Gramineae)
. Canadian Journal of Botany, 47(1): 15-21. DOI:10.1139/b69-003 |
Björkman O, 1976. Adaptive and genetic aspects of C4 photosynthesis. In: Burris RH, Black CC (eds.). Proceedings of the Fifth Annual Harry Steenbock Symposium, Madison, Wisconsin. Baltimore, MD, USA: University Park Press, pp. 287–309.
|
Black CC. 1971. Ecological implications of dividing plants into groups with distinct photosynthetic production capacities. Advances in Ecological Research, 7: 87-114. DOI:10.1016/S0065-2504(08)60203-2 |
Black E, Vidale PL, Verhoef A, et al. 2012. Cultivating C4 crops in a changing climate: sugarcane in Ghana
. Enviromental Research Letters, 7: 1-10. DOI:10.1088/1748-9326/7/4/044027 |
Blankenship RE. 1992. Origin and early evolution of photosynthesis. Photosynthesis Research, 33(2): 91-111. DOI:10.1007/BF00039173 |
Blankenship RE. 2010. Early evolution of photosynthesis. Plant Physiology, 154(2): 434-438. DOI:10.1104/pp.110.161687 |
Boom A, 2004. A geochemical study of lacustrine sediments: towards palaeo-climatic reconstructions of high Andean biomes in Colombia. Amsterdam: University of Amsterdam, pp. 125.
|
Botha CEJ. 1992. Plasmodesmatal distribution, structure and frequency in relation to assimilation in C3 and C4 grasses in southern Africa
. Planta, 187(3): 348-358. DOI:10.1007/BF00195658 |
Bowes G, Ogren WL. 1972. Oxygen inhibition and other properties of soybean ribulose 1, 5-diphosphate carboxylase. Journal of Biological and Chemical, 247(7): 2171-2176. |
Brown HA, 1999. Agronomic implications of C4 photosynthesis. In: Sage RF, Monson RK (eds.). C4 Plant Biology. San Diego, CA: Academic Press, pp. 473–508.
|
Bruhl JJ, Wilson KL. 2007. Towards a comprehensive survey of C3 and C4 photosynthetic pathways in Cyperaceae
. Aliso: A Journal of Systematic and Evolutionary Botany, 23(1): 99-148. DOI:10.5642/aliso.20072301.11 |
Carolin RC, Jacobs SWL, Vesk M. 1973. The structure of the cells of the mesophyll and parenchymatous bundle sheath of the Gramineae. Botanical Journal of the Linnean Society, 66(4): 259-275. DOI:10.1111/j.1095-8339.1973.tb02174.x |
Chollet R, 1976. C4 Control of photorespiration: studies with isolated mesophyll cells and bundle sheath strands. In: Burris RH, Black CC (eds.). CO2 Metabolism and Plant Productivity. Baltimore, Md: University Park Press, pp. 327–341.
|
Cornet D, Bonhomme JSR. 2007. Characterization of the photosynthetic pathway of some tropical food yams (Dioscorea spp.) using leaf natural 13C abundance
. Photosynthetica, 45(2): 303-305. DOI:10.1007/s11099-007-0050-0 |
Crookston RK, Moss DN. 1974. Interveinal distance for carbohydrate transport in leaves of C3 and C4 grasses
. Crop Sciences, 14(1): 123-125. DOI:10.2135/cropsci1974.0011183X001400010038x |
Dengler NG, Dengler RE, Donnelly PM, et al. 1994. Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): bundle sheath and mesophyll surface area relationships
. Annals of Botany, 73(3): 241-255. DOI:10.1006/anbo.1994.1029 |
Dengler NG, Nelson T, 1999. Leaf structure and development in C4 plants. In: Sage RF, Monson RK (eds.). C4 Plant Biology. San Diego: Academic Press, pp. 133–172.
|
Dohleman FG, Long SP. 2009. More productive than maize in the Midwest: how does Miscanthus do it?
. Plant Physiology, 150(4): 2104-2115. DOI:10.1104/pp.109.139162 |
Downes RW, Hesketh JD. 1968. Enhanced photosynthesis at low oxygen concentrations: differential response of temperate and tropical grasses. Planta, 78(1): 79-84. DOI:10.1007/BF00384860 |
Downton WJS, Tregunna EB. 1968. Carbon dioxide compensation-its relation to photosynthetic Carboxylation reactions, systematics of the Gramineae, and leaf anatomy. Canadian Journal of Botany, 46(3): 207-215. DOI:10.1139/b68-035 |
Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmospheric CO2, and climate
. Oecologia, 112(3): 285-299. DOI:10.1007/s004420050311 |
Faniyan MM, Olatunde DO, Ayeni MA, et al. 2013. Functional leaf anatomical characters in relation to C3 and C4 photosynthetic pathways in four species of Euphorbia L. in Southwestern Nigeria
. Nigerian Journal of Botany, 26(1): 19-28. |
Fisher DD, Schenk HJ, Thorsch JA, et al. 1997. Leaf anatomy and subgeneric affiliations of C3 and C4 species of Suaeda (Chenopodiaceae) in North America
. American Journal of Botany, 84(9): 1198-1210. |
Flexas J, Bota J, Loreto F, et al. 2004. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants
. Plant Biology, 6(3): 269-279. DOI:10.1055/s-2004-820867 |
Furbank RT, Taylor WC. 1995. Regulation of photosynthesis in C3 and C4 plants: a molecular approach
. The Plant Cell, 7(7): 797-807. DOI:10.1105/tpc.7.7.797 |
Furbank RT, 2009. Photosynthesis research and its application to yield potential. In: Proceedings of a Workshop Held at the Australian National University, Canberra, Australian Capital Territory, Australia. Canberra: ACIAR, pp. 14–19.
|
Gallaher RN, Ashley DA, Brown RH. 1975. 14C-photosynthate translocation in C3 and C4 plants as related to leaf anatomy
. Crop Science, 15(1): 55-59. DOI:10.2135/cropsci1975.0011183X001500010016x |
Gentry AH, 1993. A field guide to the families and genera of woody plants of northwest South America (Colombia, Ecuador and Peru): with supplementary notes on herbaceous taxa. Washington D.C.: Conservation International.
|
Ghannoum O. 2009. C4 photosynthesis and water stress
. Annals of Botany, 103(4): 635-644. DOI:10.1093/aob/mcn093 |
Hatch MD, Slack CR, Johnson HS. 1967. Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugar-cane and its occurrence in other plant species. Biochemical Journal, 102(2): 417-422. DOI:10.1042/bj1020417 |
Hatch MD. 1987. C4 photosynthesis: a unique elend of modified biochemistry, anatomy and ultrastructure
. Biochimica et Biophysica Acta (BBA)-Reviews on Bioenergetics, 895(2): 81-106. DOI:10.1016/S0304-4173(87)80009-5 |
Hattersley PW, Watson L, Johnston CR, et al. 1982. Remarkable leaf anatomical variations in Neurachne and its allies (Poaceae) in relation to C3 and C4 photosynthesis
. Botanical Journal of the Linnean Society, 84(4): 265-272. DOI:10.1111/j.1095-8339.1982.tb00364.x |
Hibberd JM, Quick WP. 2002. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants
. Nature, 415(6870): 451-454. DOI:10.1038/415451a |
Hibberd JM, Sheehy JE, Langdale JA. 2008. Using C4 photosynthesis to increase the yield of rice—rationale and feasibility
. Current Opinion in Plant Biology, 11(2): 228-231. DOI:10.1016/j.pbi.2007.11.002 |
Huxman TE, Monson RK. 2003. Stomatal responses of C3, C3-C4 and C4 Flaveria species to light and intercellular CO2 concentration: implications for the evolution of stomatal behaviour
. Plant, Cell and Environment, 26(2): 313-322. DOI:10.1046/j.1365-3040.2003.00964.x |
Jørgensen PM, Ulloa UC. 1994. Seed plants of the high Andes of Ecuador–a checklist. AAU Reports, 34: 336-355. |
Kanai R, Edward GE, 1999. The biochemistry of C4 photosynthesis. In: Sage RF, Monson RK (eds.). C4 Plant Biology. San Diego: Santiago Academic Press, pp. 49–87.
|
Kim CY, 2012. Stomatal responses of C3 and C4 Cyprus species (Cyperaceae) in Korea to Elevated CO2 concentration. Seoul, Korea: Sungshin Women's University, pp. 203.
|
Krapp A, Hofman B, Schäfer C, et al. 1993. Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the 'sink regulation' of photosynthesis?. The Plant Journal, 3(6): 817-828. DOI:10.1111/j.1365-313X.1993.00817.x |
Kruger EL, Volin JC. 2006. Reexamining the empirical relation between plant growth and leaf photosynthesis. Functional Plant Biology, 33(5): 421-429. DOI:10.1071/FP05310 |
Lal A, Edwards GE. 1996. Analysis of inhibition of photosynthesis under water stress in the C4 species Amaranthus cruentus and Zea mays: electron transport, CO2 fixation and carboxylation capacity
. Functional Plant Biology, 23(4): 403-412. |
Langdale JA. 2011. C4 cycles: past, present, and future research on C4 photosynthesis
. Plant Cell, 23(11): 3879-3892. DOI:10.1105/tpc.111.092098 |
Larcher W, 1995. Physiological Plant Ecology. New York: Springer-Verlag, pp. 506.
|
Larridon I, Huygh W, Reynders M, et al. 2011. Nomenclature and typification of names of genera and subdivisions of genera in Cypereae (Cyperaceae): 2. Names of subdivisions of Cyperus. Taxon, 60(3): 868-884. |
Leonardos ED, Grodzinski B. 2000. Photosynthesis, immediate export and carbon partitioning in source leaves of C3, C3-C4 intermediate, and C4 Panicum and Flaveria species at ambient and elevated CO2 levels
. Plant, Cell and Environment, 23(8): 839-851. DOI:10.1046/j.1365-3040.2000.00604.x |
Liu F, Stützel H. 2004. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress
. Scientia Horticulturae, 102(1): 15-27. DOI:10.1016/j.scienta.2003.11.014 |
Lloyd J, Farquhar GD. 1994. 13C discrimination during CO2 assimilation by the terrestrial biosphere
. Oecologia, 99(3–4): 201-215. DOI:10.1007/BF00627732 |
Lush WM. 1976. Leaf structure and translocation of dry matter in a C3 and a C4 grass
. Planta, 130(3): 235-244. DOI:10.1007/BF00387827 |
Marshall DM, Muhaidat M, Brown NJ, et al. 2007. Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis
. The Plant Journal, 51(5): 886-896. DOI:10.1111/j.1365-313X.2007.03188.x |
Martins S, Alves M. 2009. Anatomical features of species of Cyperaceae from Northeastern Brazil. Brittonia, 61(2): 189-200. DOI:10.1007/s12228-009-9073-0 |
McKown AD, Cochard H, Sack L. 2010. Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. The American Naturalist, 175(4): 447-460. DOI:10.1086/650721 |
Monsi M, Saeki T. 2005. On the factor light in plant communities and its importance for matter production. Annals of Botany, 95(3): 549-567. DOI:10.1093/aob/mci052 |
Muasya AM, Vrijdaghs A, Simpson DA, et al. 2009. What is a genus in Cypereae: phylogeny, character homology assessment and generic circumscription in Cypereae. The Botanical Review, 75(1): 52-66. DOI:10.1007/s12229-008-9018-4 |
Muhaidat R, Sage RF, Dengler NG. 2007. Diversity of Kranz anatomy and biochemistry in C4 eudicots
. American Journal of Botany, 94(3): 362-381. DOI:10.3732/ajb.94.3.362 |
Nelson EA, Sage TL, Sage RF. 2005. Functional leaf anatomy of plants with crassulacean acid metabolism. Functional Plant Biology, 32(5): 409-419. DOI:10.1071/FP04195 |
Nelson T, Dengler N. 1997. Leaf vascular pattern formation. The Plant Cell, 9(7): 1121-1135. DOI:10.1105/tpc.9.7.1121 |
Ogren WL, 1976. Search for higher plants with modifications of the reductive pentose phosphate pathway of CO2 assimilation. In: Burris RH, Black CC (eds.). CO2 Metabolism and Plant Productivity. Baltimore, Md.: University Park Press, pp. 19–29.
|
Oguro HO, Hinata K, Tsunoda S. 1985. Comparative anatomy and morphology of leaves between C3 and C4 species in panicum
. Annals of Botany, 55(6): 859-867. DOI:10.1093/oxfordjournals.aob.a086967 |
Ortiz-Lopez A, Ort DR, Boyer JS. 1991. Photophosphorylation in attached leaves of Helianthus annuus at low water potentials
. Plant Physiology, 96(4): 1018-1025. DOI:10.1104/pp.96.4.1018 |
Osborne CP, Freckleton RP. 2009. Ecological selection pressures for C4 photosynthesis in the grasses
. Proceedings of Royal Society, 276(1663): 1753-1760. DOI:10.1098/rspb.2008.1762 |
Park R, Epstein S. 1960. Carbon isotope fractionation during photosynthesis. Geochimica et Cosmochimica Acta, 21(1–2): 110-126. DOI:10.1016/S0016-7037(60)80006-3 |
Parry MAJ, Madgwick PJ, Carvalho JFC, et al. 2007. Prospects for increasing photosynthesis by overcoming the limitations of Rubisco. Journal of Agricultural Sciences, 145(1): 31-43. DOI:10.1017/S0021859606006666 |
Pingali PI, 2001. CIMMYT 1999–2000 world maize facts and trends. Meeting the World Maize Needs: Technological Opportunities and Priorities for the Public Sector. Mexico City: CIMMYT.
|
Raines CA. 2006. Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle
. Plant, Cell and Environment, 29(3): 331-339. DOI:10.1111/j.1365-3040.2005.01488.x |
Renvoize SA. 1987. A survey of leaf-blade anatomy in grasses XI. Paniceae. Kew Bulletin, 42(3): 739-768. DOI:10.2307/4110087 |
Ripley BS, Gilbert ME, Ibrahim DG, et al. 2007. Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata
. Journal of Experimental Botany, 58: 1351-1363. DOI:10.1093/jxb/erl302 |
Sack L, Holbrook NM. 2006. Leaf hydraulics. Annual Review of Plant Biology, 57: 361-381. DOI:10.1146/annurev.arplant.56.032604.144141 |
Sage RF. 2004. The evolution of C4 photosynthesis
. New Phytologist, 161(2): 341-370. DOI:10.1111/j.1469-8137.2004.00974.x |
Sage RF, Christin PA, Edwards EJ. 2011. The C4 plant lineages of planet earth
. Journal of Experimental Botany, 62(9): 3155-3169. DOI:10.1093/jxb/err048 |
Saiz G, Bird M, Wurster C, et al. 2015. The influence of C3 and C4 vegetation on soil organic matter dynamics in contrasting semi-natural tropical ecosystems
. Biogeosciences, 12: 5041-5059. DOI:10.5194/bg-12-5041-2015 |
Smith BN, Epstein S. 1971. Two categories of 13C/12C ratios for higher plants
. Plant Physiology, 47(3): 380-384. DOI:10.1104/pp.47.3.380 |
Somerville C, Youngs H, Taylor C, et al. 2010. Feedstocks for lignocellulosic biofuels. Science, 329: 790-792. DOI:10.1126/science.1189268 |
Soros CL, Dengler NG. 1998. Quantitative leaf anatomy of C3 and C4 Cyperaceae and comparisons with the Poaceae
. International Journal of Plant Science, 159(3): 480-491. DOI:10.1086/297565 |
Stock WD, Chuba D, Verboom GA. 2004. Distribution of South African C3 and C4 species of Cyperaceae in relation to climate and phylogeny
. Austral Ecology, 29(3): 313-319. DOI:10.1111/j.1442-9993.2004.01368.x |
Swap RJ, Aranibar JN, Dowty PR, et al., 2004. Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Global Change Biology (2004). 10: 350–358. DOI: 10.1111/j.1365-2486.2003.00702.x.
|
Taylor SH, Hulme SP, Rees M. 2010. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment
. New Phytologist, 185(3): 780-791. DOI:10.1111/j.1469-8137.2009.03102.x |
Troughton JH, Card KA, Hendy CH, 1974. Photosynthetic pathways and carbon isotope discrimination by plants. In: McGough SA (ed.). Carnegie Institute of Washington, Yearbook 73. Baltimore, Md.: Lucas Printing Co., pp. 768–780.
|
Ueno O, Sentoku N. 2006. Comparison of leaf structure and photosynthetic characteristics of C3 and C4 Alloteropsis semialata subspecies
. Plant, Cell and Environment, 29(2): 257-268. DOI:10.1111/j.1365-3040.2005.01418.x |
Ueno O, Kawano Y, Wakayama M, et al. 2006. Leaf vascular systems in C3 and C4 grasses: a two-dimensional analysis
. Annals of Botany, 97(4): 611-621. DOI:10.1093/aob/mcl010 |
Vogel JC, Fuls A, Ellis RP. 1978. The geographical distribution of Kranz grasses in South Africa. South African Journal of Science, 74: 209-217. |
Voznesenskaya EV, Franceschi VR, Kiirats O, et al. 2001. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis
. Nature, 414(6863): 543-546. DOI:10.1038/35107073 |
Voznesenskaya EV, Franceschi VR, Kiirats O, et al. 2002. Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae)
. The Plant Journal, 31(5): 649-662. DOI:10.1046/j.1365-313X.2002.01385.x |
Waller SS, Lewis JK. 1979. Occurrence of C3 and C4 photosynthetic pathways in North American grasses
. Journal of Range Management, 32(1): 12-28. DOI:10.2307/3897378 |
Wang DF, Portis AR, Moose SP, et al. 2008. Cool C4 photosynthesis: pyruvate Pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus giganteus
. Plant Physiology, 148: 557-567. DOI:10.1104/pp.108.120709 |
Welkie GW, Caldwell M. 1970. Leaf anatomy of species in some dicotyledon families as related to the C3 and C4 pathways of carbon fixation
. Canadian Journal of Botany, 48(12): 2135-2146. DOI:10.1139/b70-309 |
Whelan T, Sackett WM, Benedict CR. 1973. Enzymatic fractionation of carbon isotopes by phosphoenolpyruvate carboxylase from C4 plants
. Plant Physiology, 51(6): 1051-1054. DOI:10.1104/pp.51.6.1051 |
Winter K, Troughton JH, Evenari M, et al. 1976. Mineral ion Composition and occurrence of CAM-like diurnal malate flunctuation in plants of coastal and desert habitats of Isreal and the Sinai. Flora, 167: 1-34. |
Xoconstle-Cazáres B, Ramírez-Ortega FA, Flores-Elenes L, et al. 2010. Drought tolerance in crop plants. American Journal of Plant Physiology, 5(5): 214-256. DOI:10.3923/ajpp.2010.241.256 |
Yoshimura Y, Kubota F, Ueno O. 2004. Structural and biochemical bases of photorespiration in C4 plants: quantification of organelles and glycine decarboxylase
. Planta, 220(2): 307-317. DOI:10.1007/s00425-004-1335-1 |
Zhang JX, Kirkham MB. 1995. Water relations of water-stressed, split-root C4 (Sorghum bicolor; Poaceae) and C3 (Helianthus annuus; Asteraceae) plants
. American Journal of Botany, 82(10): 1220-1229. DOI:10.2307/2446244 |
 2017, Vol. 9
2017, Vol. 9
