2. Key Laboratory of Adaptation and Evolution of Plateau Biota, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining, Qinghai 810008, China;
3. Key Laboratory of Alpine Ecology and Biodiversity, Institute of Tibetan Plateau Research, Chinese Academy of Sciences, Beijing 100085, China;
4. CAS Center for Excellence in Tibetan Plateau Earth Science, Beijing 100101, China
Livestock grazing is a dominant land-use activity on the Tibetan Plateau, which is very sensitive to anthropogenic perturbation (Yang et al., 2013 ). Over past decades, overgrazing activity has led to severe grassland degradation on the plateau (Zhao and Zhou, 1999; Harris, 2010; Li et al., 2013b ). The Chinese government has widely implemented fencing to prevent further grassland degradation in this region. Many previous studies focused only on grazing effects on C and N biogeochemical cycling (Cao et al., 2004 ; Saggar et al., 2007 ; Wolf et al., 2010 ; Wei et al., 2012 ), while fencing effects were generally overlooked. Previous studies have reported inconsistent or even contradictory grazing effects on CH4 uptake by grasslands (Wang et al., 2005 ; Allard et al., 2007 ). Although a few studies have confirmed that grazing exclusion can improve vegetation productivity and soil quality in the Tibetan alpine meadow (Wu et al., 2009 , 2010), uncertainties still remain about the effect of grazing exclusion on the fluxes of carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) in the Tibetan alpine meadow.
Many studies have found that grazing can affect the exchange rate of CO2, CH4, and N2O between terrestrial soil and the atmosphere across many ecosystems (Allard et al., 2007 ; Saggar et al., 2007 ; Lin et al., 2009 ; Wolf et al., 2010 ; Chen et al., 2011 ; Schönbach et al., 2012 ; Wei et al., 2012 ; Falk et al., 2015 ). Grazing's direct and indirect effects on CO2, CH4, and N2O fluxes can be ascribed to several channels: (1) vegetation removal changes C and N allocation between above- and belowground biomass (Falk et al., 2015 ); (2) excreta deposition brings a high addition of readily available C and N into the soil (Maljanen et al., 2007 ; Saggar et al., 2007 ; Lin et al., 2009 ); (3) changes in the properties of plant communities modify soil microbial activity (Saggar et al., 2004 ; Maljanen et al., 2007 ) and their functional structure (Yang et al., 2013 ; Xie et al., 2014 ); and (4) vegetation removal and treading alter soil environmental conditions (e.g., soil temperature and moisture) and aeration (Klein et al., 2005 ; Wang et al., 2009 ). However, it is very difficult to separate one from the other because their effects are aggregated. Therefore, there is still no consensus on whether livestock exclusion has benefits in reducing fluxes in greenhouse gases, based on the present conflicting results of grazing on the Tibetan Plateau (Hu et al., 2010 ; Lin et al., 2011 ; Wei et al., 2012 ; Luo et al., 2013 ; Chen et al., 2016 ; Zhao et al., 2016 ).
Usually, the Tibetan alpine meadow is grazed as summer pasture and winter pasture by Tibetan sheep (Ovis aries) and yaks (Bos grunniens). Summer pastures are mostly situated along mountain slopes, with higher elevations grazed in the warm season (June–October); and winter pastures located at lower-elevation areas near the settlements of pastoralists are grazed in the cold season (November–May) (Zhao and Zhou, 1999). There are many differences between summer and winter pastures, such as plant biomass, plant composition, and labile soil-carbon content (Wang et al., 2007 ; Hu et al., 2016a ; Hu et al., 2017 ). Despite the wide study of grazing effects on CO2, CH4, and N2O fluxes on the Tibetan Plateau (Cao et al., 2004 ; Lin et al., 2009 , 2011, 2015; Hu et al., 2010 ; Wei et al., 2012 ), little is known about how these fluxes respond to grazing exclusion in summer and winter alpine meadow pastures with current grazing intensity. In this study, we measured CO2, CH4, and N2O fluxes on the fenced and grazed alpine meadow pastures situated along a slope of the Qilian Mountains on the northeastern Tibetan Plateau during two growing seasons, from May to October in 2008 and 2009. The objectives of this study were to (1) evaluate the effect of grazing exclusion on Re, CH4, and N2O fluxes in summer and winter pastures, and (2) examine the relationships of Re, CH4, and N2O fluxes with environmental factors.
2 Materials and methods 2.1 Site description and experimental designThe study was conducted at the Haibei Alpine Meadow Ecosystem Research Station (37°37′N, 101°12′E) of the Chinese Academy of Sciences, located in the northeastern part of the Tibetan Plateau. The region experiences a typical continental climate, characterized by a short and cool summer and a severely cold and long winter. The mean annual air temperature and precipitation were −1.7 °C and 570 mm, with a maximum monthly mean temperature of 10 °C in July and a minimum of −15 °C in January. About 80% of the annual precipitation falls during the growing season, from May to September (Zhao and Zhou, 1999). The soil is a clay–loam texture and classified as Gelic Cambisols according to the Food and Agriculture Organization (FAO) of the United States classification system and Mat Cry-gelic Cambisols according to the Chinese national soil-survey classification system.
Our study consisted of four vegetation types at different sites along a slope of the Qilian Mountains, with graminoid vegetation at 3,200 m (37°36′42.3″N, 101°18′47.9″E), shrub vegetation at 3,400 m (37°39′55.1″N, 101°19′52.7″E), forb vegetation at 3,600 m (37°41′46.0″N, 101°21′33.4″E), and sparse vegetation at 3,800 m a.s.l. (37°42′17.7″N, 101°22′09.2″E) in elevation. These sites were distributed within 9 km and showed differences in plant composition (Wang et al., 2014 ), biomass, and soil physicochemical properties (Table 1). Detailed information about vegetation composition can be found in Zhang et al. (2011) . All sites were grazed by Tibetan sheep and yaks for decades as a summer (December to April) or winter pasture (May to September). Graminoid vegetation is a winter pasture (Cui et al., 2014 ). Shrub, forb, and sparse vegetations are summer pastures (Zhao et al., 2006 ; Yang et al., 2013 ).
|
|
Table 1 Characteristics of selected sites for fencing and grazing along the slope of the Qilian Mountains in the northeastern part of the Tibetan Plateau. Means±Se are shown. Different lower-case letters indicate a significant difference between fencing and grazing for each vegetation type |
Three plots of 1m×1m size were fenced at 3,200 and 3,400 m in 1993 and 1998, and at 3,600 and 3,800 m in 2006 to exclude livestock grazing, respectively. Three adjacent sites of the same size continued to be open for livestock grazing.
2.2 Measurements of Re, CH4, and N2O fluxesDuring the growing seasons, from May to September in 2008 and 2009, the fluxes of Re, CH4, and N2O were measured using static chambers and gas chromatography techniques. All samplings were implemented between 09:00 a.m. and 11:00 a.m. local time on the same day every 7~10 days, depending on weather conditions (measurements were delayed due to the heavy rain events and continued when the rain stopped). The dimension and structure of chambers, method of gas sampling, and analysis were reported in detail by Lin et al. (2009) . In brief, chambers were closed for 30 minutes, and four gas samples (about 100 mL each) were manually taken from the closed chamber every 10 minutes, using 100-mL plastic syringes. The CO2, CH4, and N2O concentrations of all gas samples were analyzed by using gas chromatography (HP Series 4890D, Hewlett Packard, USA) within 24 hours after sampling.
2.3 Soil temperature and soil moistureWhen gas samples were collected, soil temperature at the 5-cm depth in fenced and grazed treatments of each site was measured using digital thermometers (JM624 digital thermometer, Living–Jinming Ltd., China); and volumetric soil moisture (%) was measured around the plots to avoid artificial disturbance, using a Time Domain Reflectometer (JS-TDR300, Meridian Measurement, USA).
2.4 Soil properties, aboveground and belowground biomassIn August of 2009, we clipped ten 0.5m×0.5m quadrats inside and outside the fence at each site to estimate aboveground biomass. These quadrats at each site were distributed every other 1 m along an east–west line. In the center of each quadrat, belowground biomass within 0~20-cm depth was collected using an 8-cm-diameter soil auger. In the laboratory, all soil cores were sieved through a 2-mm mesh and washed to remove soil and then dried at 80 °C for about 48 hours to a constant weight. Soil-bulk density was randomly determined by using cutting rings (height 5 cm, inner diameter 5 cm, volume 100 cm3); soil samples were ovendried at 105 °C and weighed. We also used a soil-drill sampler with an 8-cm diameter to take 0~10- and 10~20-cm soil samples at the center of each quadrat. These soil samples were immediately sieved through a 2-mm screen, and any visible plant materials were manually removed from the sieved soil. One part of the soil was immediately stored in an ice chest until it was taken to the laboratory and stored in a refrigerator at 4 °C prior to analyses of NH4+-N and NO3−-N. The other part of the soil was air-dried for measurement of total organic carbon, total nitrogen, and total phosphorus. The root samples were washed to remove residual soil. All plant and root samples were ovendried at 80 °C and weighed.
Soil NH4+-N and NO3−-N were extracted by shaking the soil (5 g) in 25 mL of 2 mol/L KCl within a day or two, and filtered using Whatman #40 filter paper. Their concentrations in the KCl extracts were determined on an automated segmented-flow analyzer (San++, Skalar Analytical, B.V., Netherlands). Soil total organic carbon and total nitrogen were determined by a TOC-5000A analyzer (Shimadzu Corp, Kyoto, Japan) and a Vario EL III Elemental Analyzer (Elementar, Hanau, Germany), respectively.
2.5 Statistical analysesNormality of the fluxes of Re, CH4, and N2O was tested in a One-Sample Kolmogorov-Smirnov test using SPSS version 16.0 (SPSS Inc. Chicago, USA). Repeated-measures analysis of variance (ANOVA) was used to test the effects of grazing exclusion on Re, CH4, and N2O fluxes, with fencing and vegetation as between-subject factors and with sampling date and year as within-subject factors. One-way ANOVA and least significance difference (LSD) tests were applied to determine the significance of differences in daily, monthly, and average seasonal Re, CH4, and N2O fluxes, as well as global warming potentials (GWP), between fencing and grazing for each vegetation type. Simple correlation analysis was used to test the relationships between daily Re, CH4, and N2O fluxes, and soil temperature and soil moisture. Stepwise-regression analysis was performed to test the possible dependency of average Re, CH4, and N2O fluxes in 2009 on temperature, moisture, bulk density, total organic carbon, total nitrogen, the ratio of C/N, and ammonia and nitrate of soil, as well as aboveground and belowground biomass. All significances mentioned in the text were at the 0.05 level.
3 Results 3.1 Soil temperature and soil moistureThe average soil temperature during the growing season was 9.5, 7.2, 6.7, and 4.3 °C in 2008; and 10.2, 9.1, 7.3, and 5.6 °C in 2009; for graminoid, shrub, forb, and sparse vegetation with grazing, respectively. On average, fencing decreased soil temperature by 0.8, 0.2, 0.3, and 0.2 °C for graminoid, shrub, forb, and sparse vegetation, respectively, over the 2 years. The average soil moisture varied from 8.4% (sparse vegetation) to 41.6% (graminoid vegetation), and there was no significant difference between fencing and grazing across all vegetation types.
3.2 Soil properties, aboveground and belowground biomassCompared to grazing, fencing significantly reduced soil-bulk density and increased belowground biomass for forb vegetation; and it decreased NH4+-N but increased NO3−-N for shrub vegetation. Fencing increased soil total organic carbon in winter pasture (graminoid) and reduced it in summer pastures (shrub, forb, and sparse vegetation). However, although there was no significant difference between fencing and grazing in total soil nitrogen for sparse vegetation, fencing decreased it by 10.7% on average (range 4.2%~16.0%) across graminoid, shrub, and forb vegetation. Aboveground biomass for graminoid vegetation with fencing was significantly lower, and it was significantly higher than grazing for forb and sparse vegetation.
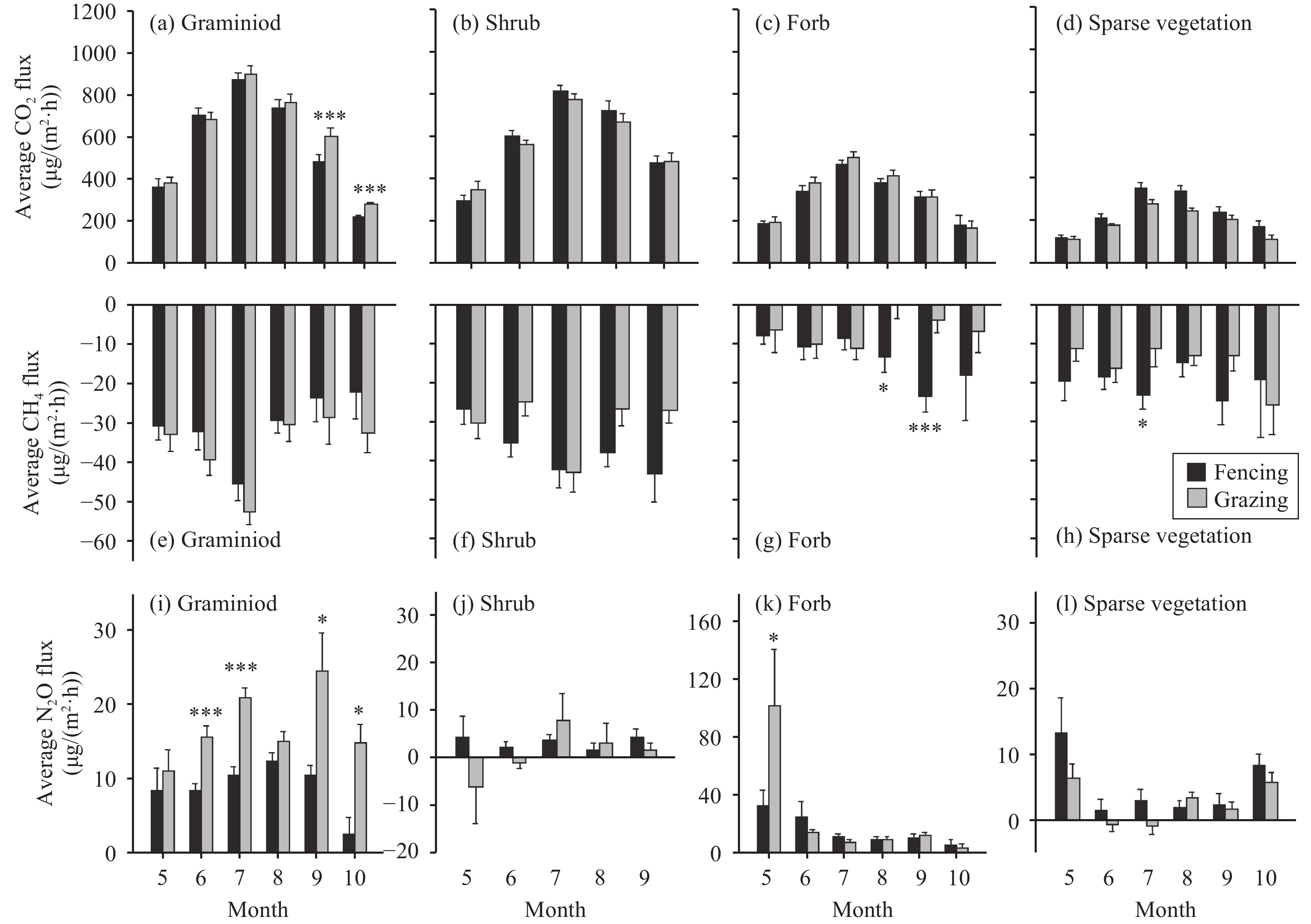
3.3 Respiration (Re)In general, sampling date, year, vegetation type, and their interactions significantly affected Re, while fencing had no effect on Re; and there was no interaction between vegetation type and fencing (Table 2). Daily Re varied from 22.1 to 1,249.0 mg/(m2·h) and peaked in July and August for all vegetation types. A significant difference in daily Re was found on several occasions, depending on vegetatione and sampling date (Figure 1). No difference in average seasonal Re was found between fencing and grazing across all vegetation types over the 2 years (Figure 2), though fencing significantly decreased monthly Re in September and October by 24.6% and 25.4% only for graminoid vegetation, respectively (Figure 4). Average seasonal Re in 2009 was 15.8% higher (range 3.5%~43.0%) across all vegetation types with fencing and grazing—except forb vegetation with fencing (13.0% lower)—than that in 2008. However, there was no difference in cumulative Re between fencing and grazing over all vegetation types over the 2 years (Table 3).
|
|
Table 2 Type III test of fixed effects on Re, CH4, and N2O fluxes in 2008 and 2009 as a summary of repeated measures from general linear model |
|
|
Table 3 Difference in cumulative Re, CH4, and N2O emission (g/m2) and global warming potentials (GWP) (g CO2 equivalent/m2) between fencing and grazing for different vegetation types in 2008 and 2009, based on GWP factor of 23 for CH4 and 296 for N2O |

|
Figure 1 Daily and average Re for graminoid (a, e), shrub (b, f), forb (c, g), and sparse vegetation (d, h), with fencing and grazing in 2008 and 2009. * and different lower-case letters indicate a significant difference between grazing and fencing at 0.05 level. Error bars indicate standard error |

|
Figure 2 Daily and average CH4 fluxes for graminoid (a, e), shrub (b, f), forb (c, g), and sparse vegetation (d, h), with fencing and grazing in 2008 and 2009. * and different lower-case letters indicate a significant difference between grazing and fencing at 0.05 level. Error bars indicate standard error |
Similar to Re, sampling date, year, vegetation type, and their interactions significantly affected CH4 fluxes; and no significant effect of fencing or interaction between fencing and vegetation type were found (Table 2). There was also no obvious variation pattern of CH4 fluxes for all vegetation types across the growing season over the 2 years. Daily CH4 fluxes varied from −76.7 to 14.5 μg/(m2·h) across all vegetation types with fencing and grazing. Although several CH4 emissions (positive value) were found, particularly for forb vegetation, all vegetation types with fencing and grazing showed daily CH4 uptake (negative value), with the averages ranging from 5.6 to 43.1 μg/(m2·h) during the growing season over the 2-year period. On the monthly scale, fencing significantly increased CH4 uptake for forbs vegetation in August and September and for sparse vegetation in July (Figure 4). Average seasonal CH4 fluxes showed no significant difference between fencing and grazing for graminoid, shrub, and sparse vegetation over the 2 years. However, compared with grazing, fencing increased average seasonal CH4 uptake for forb vegetation by 49.6% and 139.6% in 2008 (P<0.05) and 2009 (P=0.08), respectively (Figure 2). There was also no difference in cumulative CH4 fluxes between fencing and grazing or in average seasonal CH4 fluxes between 2008 and 2009 for all vegetation types (Table 3).
|
Figure 4 Average monthly fluxes of Re, CH4, and N2O during the growing season for graminoid, shrub, forb, and sparse vegetation, with fencing and grazing over 2008 and 2009. * and *** indicate a significant difference at 0.05 and 0.01 level, respectively. Error bars indicate standard error |
N2O fluxes were generally affected by sampling date, year, vegetation type, and their interactions. Fencing had no effect on daily N2O fluxes, and there was no interaction between fencing and vegetation type (Table 2). Daily N2O fluxes highly varied, in the range from −97.1 to 536.4 μg/(m2·h), and showed no clear variation pattern for all vegetation types across the growing season over the 2 years. Both N2O emission (positive values) and uptake (negative values) were observed across all vegetation types over the 2 years. Several significant differences in daily N2O fluxes between fencing and grazing were found only for graminoid vegetation (Figure 3). As a result, fencing reduced its monthly N2O fluxes by 15% on average (range 6.7%~31.7%), compared with grazing over the 2 years. Based on the seasonal N2O fluxes and accumulative N2O fluxes, a significant difference between fencing and grazing was found only for graminoid vegetation over the 2 years (Figure 2, Table 3). Average seasonal N2O fluxes for graminoid vegetation with fencing in 2008 were significantly higher than that in 2009, while they were significantly lower for forb vegetation with fencing and grazing than that in 2009.

|
Figure 3 Daily and average N2O fluxes for graminoid (a, e), shrub (b, f), forb (c, g), and sparse vegetation (d, h), with fencing and grazing in 2008 and 2009. * and different lower-case letters indicate a significant difference between grazing and fencing at 0.05 level. Error bars indicate standard error |
Generally, Re was significantly positively related to soil temperature, soil moisture, soil total organic C, the ratio of C/N, nitrate, aboveground biomass, and belowground biomass; and negatively correlated with soil-bulk density and ammonia. Soil temperature, soil moisture, soil-bulk density, and soil total organic C explained approximately 66%, 31%, 28%, and 45% of the variation in daily or average seasonal Re over the 2 years, respectively. Other biotic factors explained approximately 19%~71% of average seasonal Re in 2009 across all vegetation types. The effect of soil temperature on daily CH4 fluxes was very weak (R2=0.06), even though the relationship was significant. Daily CH4 fluxes were significantly and positively related to soil moisture, indicating that higher soil moisture could restrain CH4 uptake. Soil moisture explained 14% of variation of daily CH4 fluxes over the 2 years. In comparison, aboitic factors—including soil total organic C, the ratio of C/N, ammonia, nitrate, and aboveground biomass explained more (20%~33%) variation in average seasonal CH4 fluxes. Significantly, relationship was found only between N2O fluxes and soil-bulk density, explaining about 27% of variation in average seasonal CH4 fluxes over 2-year (Table 4).
|
|
Table 4 Relationships between daily (A, B) or average (C, D, E, F, G, H, I) Re, CH4, and N2O fluxes with soil temperature (A), soil moisture (B), soil-bulk density (C), total organic C (D), ratio of C/N (E), ammonia (F), nitrate (G), aboveground biomass (H), and belowground biomass (I) for pooled data of all vegetation types with fencing and grazing |
To determine the relative control on Re, CH4, and N2O fluxes from biotic and abiotic factors, we analyzed stepwise regressions between average seasonal Re, CH4, and N2O fluxes in 2009 and various environmental factors including total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), the ratio of C to N (C/N), ammonia and nitrate in soil, aboveground and belowground biomass, and abiotic factors (average soil temperature, soil moisture, soil-bulk density). Equations of stepwise-regression analysis for average Re and CH4 fluxes were Y=63.41T+6.32M−155.27 (R2=0.90, n=24, P<0.001) andY=0.90M−22.65BD+14.01 (R2=0.68, n=24, P<0.001), whereT, M, and BD are the temperature, moisture, and bulk density of soil, respectively. These results indicated that aboitic factors rather than biotic factors were the main ones controlling average Re and CH4 fluxes.
4 Discussion 4.1 Respiration (Re)Fencing had no effect on daily Re, and there was no significant difference in average seasonal Re between fencing and grazing during the growing season over the 2 years across all vegetation types (Figure 1); these results coincided with those of other studies (Susiluoto et al., 2008 ; Lin et al., 2011 ). Several abiotic and biotic variables affected Re, such as temperature, moisture, plant biomass, and soil organic C (SOC) (Kato et al., 2006 ; Zheng et al., 2009 ; Lin et al., 2011 ; Hu et al., 2016a ; Zhao et al., 2016 ). As in previous studies (Lin et al., 2011 ; Hu et al., 2016a ; Zhao et al., 2016 ), we also found Re to be positively related to soil temperature, soil moisture, aboveground biomass, and SOC. Grazing may enhance Re because removal of aboveground biomass by livestock increases soil temperature (Wei et al., 2012 ; Li et al., 2013a ). Moreover, fencing may increase Re because increased litter deposited on soil surface can release CO2 in the decomposition process (Cao et al., 2004 ; Yang et al., 2013 ). However, fencing can also reduce Re due to exclusion of CO2 emission from excreta patches (Ma et al., 2006 ; Lin et al., 2009 ). Mixed responses of aboveground biomass, belowground biomass, and SOC to fencing with reported increase (Wu et al., 2009 , 2010; Hafner et al., 2012 ), decrease (Shi et al., 2013 ; Cui et al., 2014 ), or no effect (Lin et al., 2011 ; Rui et al., 2011 ; Wei et al., 2012 ; Zhao et al., 2016 ) has been found in the Tibetan grassland. In our study, the positive effect of increased soil moisture to Re may be partially balanced by the negative effect of decreased soil temperature. Furthermore, fencing significantly decreased aboveground biomass for winter pasture but increased it for summer pasture (forb and sparse vegetation). In contrast, fencing significantly increased TOC in winter pasture, while it decreased TOC in summer pastures. These contrary responses in aboveground biomass and SOC to fencing probably offset their mutual effects on Re, leading to no detectable impact of fencing on average seasonal Re across all vegetation types. These results point to potentially different effects of grazing on Re in summer and winter pastures through changing allocation of C accumulation between aboveground biomass and soil. It also implies that the effect of grazing exclusion on Re in the Tibetan alpine meadow depends on the balance of positive and negative effects between biotic and abiotic factors (Lin et al., 2009 ; Chen et al., 2016 ).
4.2 CH4 uptakeSimilar to the results of previous studies (Wang et al., 2009 ; Lin et al., 2015 ), we also found the Tibetan alpine meadow with fencing and grazing acted so as to produce a net uptake of atmospheric CH4 during the growing season. The average CH4 uptake rates (23.8±0.8 μg/(m2·h)) across all vegetation types with fencing and grazing were much lower than the reported values from the Tibetan alpine steppes (63.4~70.2 μg/(m2·h)) (Wei et al., 2012 ) and typical grassland in Inner Mongolia (86.0 μg/(m2·h)) (Wang et al., 2005 ). Although daily CH4 fluxes were positively related to soil temperature, which is in agreement with other studies (Lin et al., 2015 ; Hu et al., 2016b ), their relationship was very weak (R2=0.06). Thus, there is no clear seasonal variation pattern with temperature in daily CH4 fluxes across all vegetation types. Similar to other reports (Wei et al., 2012 ; Lin et al., 2015 ), we also found that CH4 uptake was negatively related to soil moisture (Table 4). Thus, the lowest average CH4 uptake for forb vegetation (9.7±0.8 μg/(m2·h)) can be ascribed to its high soil moisture. Probably, high soil moisture not only blocks diffusion of atmospheric oxygen and CH4 into the soil but also depresses the oxidation ability of methanotrophs and benefits methanogens to produce CH4, due to anaerobic soil conditions (Zhuang et al., 2013 ).
Some studies show that fenced pasture can uptake more atmospheric CH4 than grazed pasture (Liu et al., 2007 ; Saggar et al., 2007 ; Wei et al., 2012 ) because grazing exclusion may decrease soil-bulk density due to eliminating soil compaction from livestock trampling (Wei et al., 2012 ), increase the population of CH4 oxidation bacteria (Liu et al., 2007 ; Wang et al., 2016 ), relieve competitive inhibition of NH4+ on CH4 oxidation and toxicity of NO3− on methanotrophs (Jiang et al., 2010 ; Fang et al., 2014 ), and exclude release of CH4 from patches of dung and urine (Ma et al., 2006 ; Lin et al., 2009 ). Our study found that fencing increased average CH4 uptake for summer pastures, though there was no significant difference between fencing and grazing in most cases, which coincides with some other studies with light or moderate grazing (Chen et al., 2011 ; Lin et al., 2015 ). The lower soil-bulk density for forb vegetation with fencing may be conductive to CH4 oxidation by improving atmospheric CH4 diffusion reach methanotrophs (Striegl, 1993; Urmann et al., 2009 ), leading to a significant increase in average CH4 uptake in 2008. Another possible mechanism is that grazing can support a higher abundance of methanogenesis that could produce more CH4 in the surrounding anaerobic soil conditions (Yang et al., 2013 ). The lower temperature due to litter accumulation likely maintains higher soil moisture in the fenced winter pasture, though no difference was found between fencing and grazing. In this case, higher soil moisture constrains atmospheric CH4 and O2 reach methanotrophs (Urmann et al., 2009 ), which might respond positively to the temperature increase (Pearce and Clymo, 2001; Zheng et al., 2012 ). These two contrary effects on CH4 uptake might be responsible for the finding of no significant difference between fencing and grazing regardless of summer and winter pastures, suggesting that grazing exclusion may have a weak impact on CH4 uptake in the alpine meadow, compared to moderate grazing density (Lin et al., 2015 ).
4.3 N2O emissionAlthough our study found that fencing had no effect on daily N2O fluxes (Table 2), it caused a significant reduction in average seasonal N2O emission from winter pasture (Figure 1). N2O fluxes are closely related to inputs of nitrogen (Ma et al., 2006 ; Hynšt et al., 2007 ; Lin et al., 2009 ). Allard et al. (2007) concluded that N2O emission largely depends on grazing density, with higher emissions under greater grazing density. N2O emissions from grazing are mainly derived from animal excreta, caused by greater input of NH4+-N and NO3−-N into the soil (Ma et al., 2006 ; Lin et al., 2009 ), stimulating nitrification in the surrounding aerobic soil and denitrification in the anaerobic condition (Nielsen et al., 1996 ; Hynšt et al., 2007 ). In our study, winter pasture (graminoid vegetation) was covered by many patches of deposited animal excreta (Hu et al., 2010 ). Thus, despite fencing causing some changes in soil physical characteristic, nutrients, and plant biomass (Table 1), the contribution of fencing to the reduction in N2O emission from winter pasture can be mainly ascribed to the exclusion of input of NH4+-N and NO3−-N from excreta, restraining nitrification and denitrification in the soils. Most N2O emissions induced by animal excreta occur over a short period (Ma et al., 2006 ; Hynšt et al., 2007 ; Lin et al., 2009 ), but we found that significantly higher N2O emission from winter pasture can last a long time during the growing season (Figure 3). These results suggest that winter alpine pasture with many excreta patches could be an important source of N2O emission in the region.
We also found fencing did not change average N2O emission from summer pastures (Figure 1). The oxygen condition in the soil may also influence the response of N2O emission to fencing, by changing nitrification and denitrification (Rysgaard et al., 1994 ; Maag and Vinther, 1996). Our finding that N2O emission was negatively related to soil-bulk density indicates that fencing should reduce N2O emission, due to a better soil-oxygen condition. However, no significant difference was found between grazing and fencing for forb vegetation, though fencing decreased soil-bulk density. Xu et al. (2003) found that nitrification is the main process contributing to N2O emission, whereas anaerobic conditions are less frequent. Fenced pasture tends to have lower nitrification than grazed pastures (Groffman et al., 1993 ; Tracy and Frank, 1998), which may also lead to reduced N2O emission. Meanwhile, fencing might create a relatively aerobic soil condition due to exclusion of animal trampling that may constrain denitrification in the soil (Maag and Vinther, 1996; Allard et al., 2007 ), which may be beneficial to N2O production. Therefore, the effect of fencing on N2O fluxes depends on the positive and negative effects on the N2O production process (Hu et al., 2010 ). Xie et al. (2014) found fencing can increase the abundance of both soil ammonia-oxidizing bacteria and some denitrifiers. However, fencing reduced the abundance of nitrification genes and increased denitrification genes (Yang et al., 2013 ). In light of the tradeoff effects on nitrification, denitrification, and the related microbial community, fencing did not affect average N2O emission from summer pastures in our study.
5 ConclusionsThe Tibetan alpine meadow acted as a net sink of CH4, with an average uptake rate of 23.8 μg/(m2·h), and as a source of N2O emission, at an average rate of 9.7 μg/(m2·h), during the growing season across all vegetation types. In general, daily fluxes of CO2, CH4, and N2O were significantly affected by date, year, vegetation type, and their interactions. Fencing significantly increased only the average seasonal CH4 uptake from a summer pasture (forb vegetation) in 2008 and reduced average seasonal N2O emission from the winter pasture over the 2 years, due to exclusion of livestock excreta. However, owing to small proportions of CH4 uptake and N2O emission in Re and the tradeoff of opposite responses of CH4 uptake and N2O emission to fencing, no difference in GWP was found between grazing and fencing, regardless of summer or winter pasture. These results indicate that effects of grazing exclusion, given current grazing intensity and pattern, seem to be very weak in reducing the release of greenhouse gases from the Tibetan alpine meadow.
Acknowledgments:We thank two anonymous reviewers and the editor for their constructive comments and suggestions to improve this manuscript. This work was financially supported by the Natural Science Foundation Committee of China (41230750 and 41101081), Key Program of the Chinese Academy of Sciences (KFZD-SW-312) and the "National Key Research and Development Program of China" (2016YFC0501802).
Allard V, Soussana JF, Falcimagne R, et al. 2007. The role of grazing management for the net biome productivity and greenhouse gas budget (CO2, N2O and CH4) of semi-natural grassland
. Agriculture, Ecosystems & Environment, 121(1–2): 47-58. DOI:10.1016/j.agee.2006.12.004 |
Cao GM, Tang YH, Mo WH, et al. 2004. Grazing intensity alters soil respiration in an alpine meadow on the Tibetan Plateau. Soil Biology and Biochemistry, 36(2): 237-243. DOI:10.1016/j.soilbio.2003.09.010 |
Chen J, Zhou XH, Wang JF, et al. 2016. Grazing exclusion reduced soil respiration but increased its temperature sensitivity in a Meadow Grassland on the Tibetan Plateau. Ecology and Evolution, 6(3): 675-687. DOI:10.1002/ece3.1867 |
Chen WW, Wolf B, Zheng XH, et al. 2011. Annual methane uptake by temperate semiarid steppes as regulated by stocking rates, aboveground plant biomass and topsoil air permeability. Global Change Biology, 17(9): 2803-2816. DOI:10.1111/j.1365-2486.2011.02444.x |
Cui S, Zhu X, Wang S, et al. 2014. Effects of seasonal grazing on soil respiration in alpine meadow on the Tibetan plateau. Soil Use and Management, 30(3): 435-443. DOI:10.1111/sum.12125 |
Falk JM, Schmidt NM, Christensen TR, et al. 2015. Large herbivore grazing affects the vegetation structure and greenhouse gas balance in a high arctic mire. Environmental Research Letters, 10(4): 045001. DOI:10.1088/1748-9326/10/4/045001 |
Fang HJ, Cheng SL, Yu GR, et al. 2014. Low-level nitrogen deposition significantly inhibits methane uptake from an alpine meadow soil on the Qinghai-Tibetan Plateau. Geoderma, 213: 444-452. DOI:10.1016/j.geoderma.2013.08.006 |
Groffman PM, Rice CW, Tiedje JM. 1993. Denitrification in a tallgrass prairie landscape. Ecology, 74(3): 855-862. DOI:10.2307/1940811 |
Hafner S, Unteregelsbacher S, Seeber E, et al. 2012. Effect of grazing on carbon stocks and assimilate partitioning in a Tibetan montane pasture revealed by 13CO2 pulse labeling
. Global Change Biology, 18(2): 528-538. DOI:10.1111/j.1365-2486.2011.02557.x |
Harris RB. 2010. Rangeland degradation on the Qinghai-Tibetan plateau: A review of the evidence of its magnitude and causes. Journal of Arid Environments, 74(1): 1-12. DOI:10.1016/j.jaridenv.2009.06.014 |
Hu YG, Chang XF, Lin XW, et al. 2010. Effects of warming and grazing on N2O fluxes in an alpine meadow ecosystem on the Tibetan plateau
. Soil Biology and Biochemistry, 42(6): 944-952. DOI:10.1016/j.soilbio.2010.02.011 |
Hu YG, Jiang LL, Wang SP, et al. 2016a. The temperature sensitivity of ecosystem respiration to climate change in an alpine meadow on the Tibet plateau: A reciprocal translocation experiment. Agricultural and Forest Meteorology, 216: 93-104. DOI:10.1016/j.agrformet.2015.10.002 |
Hu YG, Wang Q, Wang SP, et al. 2016b. Asymmetric responses of methane uptake to climate warming and cooling of a Tibetan alpine meadow assessed through a reciprocal translocation along an elevation gradient. Plant and Soil, 402(1–2): 263-275. DOI:10.1007/s11104-016-2791-7 |
Hu YG, Wang ZR, Wang Q, et al. 2017. Climate change affects soil labile organic carbon fractions in a Tibetan alpine meadow. Journal of Soils and Sediments, 17(2): 326-339. DOI:10.1007/s11368-016-1565-4 |
Hynšt J, Šimek M, Brůček P, et al. 2007. High fluxes but different patterns of nitrous oxide and carbon dioxide emissions from soil in a cattle overwintering area. Agriculture, Ecosystems & Environment, 120(2–4): 269-279. DOI:10.1016/j.agee.2006.10.003 |
Jiang CM, Yu GR, Fang HJ, et al. 2010. Short-term effect of increasing nitrogen deposition on CO2, CH4 and N2O fluxes in an alpine meadow on the Qinghai-Tibetan Plateau, China
. Atmospheric Environment, 44(24): 2920-2926. DOI:10.1016/j.atmosenv.2010.03.030 |
Kato T, Tang YH, Gu S, et al. 2006. Temperature and biomass influences on interannual changes in CO2 exchange in an alpine meadow on the Qinghai-Tibetan Plateau
. Global Change Biology, 12(7): 1285-1298. DOI:10.1111/j.1365-2486.2006.01153.x |
Klein JA, Harte J, Zhao XQ. 2005. Dynamic and complex microclimate responses to warming and grazing manipulations. Global Change Biology, 11(9): 1440-1451. DOI:10.1111/j.1365-2486.2005.00994.x |
Li XD, Zhang CP, Fu H, et al. 2013a. Grazing exclusion alters soil microbial respiration, root respiration and the soil carbon balance in grasslands of the Loess Plateau, northern China. Soil Science and Plant Nutrition, 59(6): 877-887. DOI:10.1080/00380768.2013.862157 |
Li XL, Gao J, Brierley G, et al. 2013b. Rangeland degradation on the Qinghai-Tibet Plateau: Implications for rehabilitation. Land Degradation & Development, 24(1): 72-80. DOI:10.1002/ldr.1108 |
Lin XW, Wang SP, Hu YG, et al. 2015. Experimental warming increases seasonal methane uptake in an alpine meadow on the Tibetan Plateau. Ecosystems, 18(2): 274-286. DOI:10.1007/s10021-014-9828-7 |
Lin XW, Wang SP, Ma XZ, et al. 2009. Fluxes of CO2, CH4, and N2O in an alpine meadow affected by yak excreta on the Qinghai-Tibetan plateau during summer grazing periods
. Soil Biology and Biochemistry, 41(4): 718-725. DOI:10.1016/j.soilbio.2009.01.007 |
Lin XW, Zhang ZH, Wang SP, et al. 2011. Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan plateau. Agricultural and Forest Meteorology, 151(7): 792-802. DOI:10.1016/j.agrformet.2011.01.009 |
Liu CY, Holst J, Brüggemann N, et al. 2007. Winter-grazing reduces methane uptake by soils of a typical semi-arid steppe in Inner Mongolia, China. Atmospheric Environment, 41(28): 5948-5958. DOI:10.1016/j.atmosenv.2007.03.017 |
Luo JF, Ledgard SF, Lindsey SB. 2013. Nitrous oxide and greenhouse gas emissions from grazed pastures as affected by use of nitrification inhibitor and restricted grazing regime. Science of the Total Environment, 465: 107-114. DOI:10.1016/j.scitotenv.2012.12.075 |
Ma XZ, Wang SP, Wang YF, et al. 2006. Short-term effects of sheep excrement on carbon dioxide, nitrous oxide and methane fluxes in typical grassland of Inner Mongolia. New Zealand Journal of Agricultural Research, 49(3): 285-297. DOI:10.1080/00288233.2006.9513719 |
Maag M, Vinther FP. 1996. Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Applied Soil Ecology, 4(1): 5-14. DOI:10.1016/0929-1393(96)00106-0 |
Maljanen M, Martikkala M, Koponen HT, et al. 2007. Fluxes of nitrous oxide and nitric oxide from experimental excreta patches in boreal agricultural soil. Soil Biology and Biochemistry, 39(4): 914-920. DOI:10.1016/j.soilbio.2006.11.001 |
Nielsen TH, Nielsen LP, Revsbech NP. 1996. Nitrification and coupled nitrification-denitrfication associated with a soil-manure interface. Soil Science Society of American Journal, 60(6): 1829-1840. DOI:10.2136/sssaj1996.03615995006000060031x |
Pearce DME, Clymo RS. 2001. Methane oxidation in a peatland core. Global Biogeochemical Cycles, 15(3): 709-720. DOI:10.1029/2000GB001323 |
Rui YC, Wang SP, Xu ZH, et al. 2011. Warming and grazing affect soil labile carbon and nitrogen pools differently in an alpine meadow of the Qinghai-Tibet Plateau in China. Journal of Soils and Sediments, 11(6): 903-914. DOI:10.1007/s11368-011-0388-6 |
Rysgaard S, Risgaard-Petersen N, Peter SN, et al. 1994. Oxygen regulation of nitrification and denitrification in sediments. Limnology and Oceanography, 39(7): 1643-1652. DOI:10.4319/lo.1994.39.7.1643 |
Saggar S, Bolan NS, Bhandral R, et al. 2004. A review of emissions of methane, ammonia, and nitrous oxide from animal excreta deposition and farm effluent application in grazed pastures. New Zealand Journal of Agricultural Research, 47(4): 513-544. DOI:10.1080/00288233.2004.9513618 |
Saggar S, Hedley CB, Giltrap DL, et al. 2007. Measured and modelled estimates of nitrous oxide emission and methane consumption from a sheep-grazed pasture. Agriculture, Ecosystems & Environment, 122(3): 357-365. DOI:10.1016/j.agee.2007.02.006 |
Schönbach P, Wolf B, Dickhöfer U, et al. 2012. Grazing effects on the greenhouse gas balance of a temperate steppe ecosystem. Nutrient Cycling in Agroecosystems, 93(3): 357-371. DOI:10.1007/s10705-012-9521-1 |
Shi XM, Li XG, Li CT, et al. 2013. Grazing exclusion decreases soil organic C storage at an alpine grassland of the Qinghai-Tibetan Plateau. Ecological Engineering, 57: 183-187. DOI:10.1016/j.ecoleng.2013.04.032 |
Striegl RG. 1993. Diffusional limits to the consumption of atmospheric methane by soils. Chemosphere, 26(1–4): 715-720. DOI:10.1016/0045-6535(93)90455-E |
Susiluoto S, Rasilo T, Pumpanen J, et al. 2008. Effects of grazing on the vegetation structure and carbon dioxide exchange of a Fennoscandian fell ecosystem. Arctic, Antarctic, and Alpine Research, 40(2): 422-431. DOI:10.1657/1523-0430(07-035) |
Tracy BF, Frank DA. 1998. Herbivore influence on soil microbial biomass and nitrogen mineralization in a northern grassland ecosystem: yellowstone National Park. Oecologia, 114(5): 556-562. DOI:10.1007/s004420050480 |
Urmann K, Lazzaro A, Gandolfi I, et al. 2009. Response of methanotrophic activity and community structure to temperature changes in a diffusive CH4/O2 counter gradient in an unsaturated porous medium
. FEMS Microbiology Ecology, 69(2): 202-212. DOI:10.1111/j.1574-6941.2009.00708.x |
Wang CT, Long RJ, Wang QF, et al. 2007. Effects of altitude on plant-species diversity and productivity in an alpine meadow, Qinhai-Tibetan Plateau. Australian Journal of Botany, 55(2): 110-117. DOI:10.1071/BT04070 |
Wang MM, Wang SP, Wu LW, et al. 2016. Evaluating the lingering effect of livestock grazing on functional potentials of microbial communities in Tibetan grassland soils. Plant and Soil, 407(1–2): 385-399. DOI:10.1007/s11104-016-2897-y |
Wang SP, Yang XX, Lin XW, et al. 2009. Methane emission by plant communities in an alpine meadow on the Qinghai-Tibetan Plateau: a new experimental study of alpine meadows and oat pasture. Biology Letters, 5(4): 535-538. DOI:10.1098/rsbl.2009.0123 |
Wang SP, Meng FD, Duan JC, et al. 2014. Asymmetric sensitivity of first flowering date to warming and cooling in alpine plants. Ecology, 95(12): 3387-3398. |
Wang YS, Xue M, Zheng XH, et al. 2005. Effects of environmental factors on N2O emission from and CH4 uptake by the typical grasslands in the Inner Mongolia
. Chemosphere, 58(2): 205-215. DOI:10.1016/j.chemosphere.2004.04.043 |
Wei D, Xu R, Wang YH, et al. 2012. Responses of CO2, CH4 and N2O fluxes to livestock exclosure in an alpine steppe on the Tibetan Plateau, China
. Plant and Soil, 359(1–2): 45-55. DOI:10.1007/s11104-011-1105-3 |
Wolf B, Zheng XH, Brüggemann N, et al. 2010. Grazing-induced reduction of natural nitrous oxide release from continental steppe. Nature, 464(7290): 881-884. DOI:10.1038/nature08931 |
Wu GL, Du GZ, Liu ZH, et al. 2009. Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau. Plant and Soil, 319(1–2): 115-126. DOI:10.1007/s11104-008-9854-3 |
Wu GL, Liu ZH, Zhang L, et al. 2010. Long-term fencing improved soil properties and soil organic carbon storage in an alpine swamp meadow of western China. Plant and Soil, 332(1–2): 331-337. DOI:10.1007/s11104-010-0299-0 |
Xie Z, Le Roux X, Wang CP, et al. 2014. Identifying response groups of soil nitrifiers and denitrifiers to grazing and associated soil environmental drivers in Tibetan alpine meadows. Soil Biology and Biochemistry, 77: 89-99. DOI:10.1016/j.soilbio.2014.06.024 |
Xu R, Wang M, Wang Y. 2003. Using a modified DNDC model to estimate N2O fluxes from semi-arid grassland in China
. Soil Biology and Biochemistry, 35(4): 615-620. DOI:10.1016/S0038-0717(03)00009-9 |
Yang YF, Wu LW, Lin QY, et al. 2013. Responses of the functional structure of soil microbial community to livestock grazing in the Tibetan alpine grassland. Global Change Biology, 19(2): 637-648. DOI:10.1111/gcb.12065 |
Zhang FW, Li YN, Cao GM, et al. 2011. Response of alpine plant community to simulated climate change: two-year results of reciprocal translocation experiment (Tibetan Plateau). Polish Journal of Ecology, 59(4): 741-751. |
Zhao JX, Luo TX, Li RC, et al. 2016. Grazing effect on growing season ecosystem respiration and its temperature sensitivity in alpine grasslands along a large altitudinal gradient on the central Tibetan Plateau. Agricultural and Forest Meteorology, 218–219: 114-121. DOI:10.1016/j.agrformet.2015.12.005 |
Zhao L, Li YN, Xu SX, et al. 2006. Diurnal, seasonal and annual variation in net ecosystem CO2 exchange of an alpine shrubland on Qinghai-Tibetan plateau
. Global Change Biology, 12(10): 1940-1953. DOI:10.1111/j.1365-2486.2006.01197.x |
Zhao XQ, Zhou XM. 1999. Ecological basis of alpine meadow ecosystem management in Tibet: Haibei alpine meadow ecosystem research station. Ambio, 28(8): 642-647. |
Zheng Y, Yang W, Sun X, et al. 2012. Methanotrophic community structure and activity under warming and grazing of alpine meadow on the Tibetan plateau. Applied Microbiology and Biotechnology, 93(5): 2193-2203. DOI:10.1007/s00253-011-3535-5 |
Zheng ZM, Yu GR, Fu YL, et al. 2009. Temperature sensitivity of soil respiration is affected by prevailing climatic conditions and soil organic carbon content: a trans-China based case study. Soil Biology and Biochemistry, 41(7): 1531-1540. DOI:10.1016/j.soilbio.2009.04.013 |
Zhuang QL, Chen M, Xu K, et al. 2013. Response of global soil consumption of atmospheric methane to changes in atmospheric climate and nitrogen deposition. Global Biogeochemical Cycles, 27(3): 650-663. DOI:10.1002/gbc.20057 |
 2017, Vol. 9
2017, Vol. 9
