2. Polar Research Institute of China, Shanghai 200136, China;
3. Climate Change Institute, University of Maine, Orono, Maine 04469, USA;
4. School of Earth and Climate Sciences, University of Maine, Orono, Maine 04469, USA
Lead, arsenic, and copper (Pb, As, and Cu, respectively) are some of the trace elements of most concern in terms of human health and environmental effects, due to their bioavailability and toxicity (Pacyna and Pacyna, 2001; Järup, 2003). They exist naturally in the earth's environment in low concentrations. However, human activities, including fossil-fuel combustion, metal smelting, industry, agriculture, and large-scale land use, can lead to higher concentrations of these trace elements than their natural background levels (Nriagu and Davidson, 1986). The global atmospheric cycle of these trace elements has been significantly changed by human activities in many regions of the Earth's surface (Chen and Graedel, 2012). These alterations are well preserved in snow and ice cores in Antarctica that can be utilized to reflect past changes of the atmospheric composition. Lead was first found in polar snow polluted notably by anthropogenic activities (Murozumi et al., 1969 ). Afterwards, a series of studies concerning snow pits and ice cores collected across Antarctica suggested that the Pb, As, and Cu concentrations showed an apparent increasing trend during the 20th century (Boutron and Patterson, 1987; Wolff and Suttie, 1994; Wolff et al., 1999 ; Planchon et al., 2002a ,b; Vallelonga et al., 2004 ; McConnell et al., 2014 ; Potocki et al., 2016 ; Schwanck et al., 2016 ). The results are consistent with the changes of human activities in the Southern Hemisphere, such as automobile-exhaust emission, mining, and nonferrous metal smelting. However, concentrations of Pb, As, and Cu in Antarctic snow and ice show wide spatiotemporal variations. For example, As concentrations of a snow pit collected at Dome Fuji showed a rapidly decreasing trend from the 1990 to the 2000, by a factor of about ten (Hong et al., 2012 ), while the results from a West Antarctic ice core showed a decreasing trend by a factor of only about two (Schwanck et al., 2016 ). In addition, Hur et al. (2007) found distinct seasonal patterns of Pb and Cu at different Antarctic sites.
Knowledge about the geographical variations of the trace elements in Antarctic snow and ice is crucial to improving our understanding of major environmental controls on the Antarctic surface-snow chemistry, sources of local and global anthropogenic pollutants, and mechanisms of their transport and deposition into Antarctic snow. Unfortunately, spatial distributions of these metals across Antarctica are focused on a limited number of sites studied. Ikegawa et al. (1999) suggested that altitude and distance from the coast were the major factors influencing the heavy-metal concentrations, while Dixon et al. (2013) found that trace-element concentrations (including Pb and As) in the surface snow of the non-glaze/dune area were generally lower than those in the dune area. Grotti et al. (2011) observed that Pb, As, and Cu showed high concentrations or enrichment factors on the inland plateau, which might be attributed to the existence of long-range transportation or polar stratospheric precipitation. Here, we present the first data set of Pb, As, and Cu concentrations of the surface-snow samples collected along the transect from the Zhongshan Station to Dome A (80°22′51″S, 77°27′23″E, 4,093 m a.s.l.) during the 2009–2010 austral summer, for the purpose of studying the distributions of Pb, As, and Cu along the transect, together with their possible sources, atmospheric transport, and deposition processes.
2 Sampling and analyses 2.1 SamplingThe transect from the Zhongshan Station to Dome A is located on the eastern side of the Lambert Glacier Basin (LGB) and the western side of Elizabeth Princess Land, approximately along the 77°E longitude line. A total of 58 samples was collected along the transect, with an interval of about 20 km (Figure 1). Sampling was carried out 50 m upwind of the vehicle tracks or camp to avoid possible local contamination. The surface snow of 10 cm was collected by pushing the sampling bottles into the snow surface. The plastic scraper and low-density polyethylene (LDPE) widemouthed bottles were thoroughly acid-cleaned prior to leaving for the field, following the strict cleaning procedures from Hong et al. (2000) and Liu et al. (2011) . All the samples were double-sealed within acid-cleaned LDPE bags and were kept frozen (−10 °C) during transportation to the State Key Laboratory of Cryospheric Science (SKLCS) and stored in a freezer (−18 °C) until analysis.
All procedures, such as tool cleaning, sampling, and transportation, were carefully monitored to prevent adverse environmental impacts and potential contamination. The plastic scraper and low-density polyethylene (LDPE) widemouthed bottles were thoroughly acid-cleaned prior to leaving for the field, following the strict cleaning procedures from Hong et al. (2000) and Liu et al. (2011) .

|
Figure 1 Map showing sampling sites (red dots) along the transect from the Zhongshan Station to Dome A |
Analyses were performed at the Climate Change Institute (CCI) at the University of Maine in dedicated ultraclean labs. The samples were melted in the ultraclean room (Class 1000) at room temperature (20 °C). Afterwards, they were acidified with 1% ultrapure nitric acid (Fisher "Optima" Grade), stored at room temperature for 48 hours, and measured by a Thermo ELEMENT2-High Resolution Inductively Coupled Plasma Sector Field Mass Spectrometer (ICP-SFMS) with an APEX micronebulization desolvation system (APEX, ESI, US), which increases instrument sensitivity and reduces oxide formation in the plasma (Osterberg et al., 2006 ). Injection was achieved using a CETAC ASX 260 autosampler, housed under a Class 100 bench to reduce possible contamination. A high-resolution setting and a sensitivity of 115In = 800,000 cps per 100 ng/L were used. The ICP-SFMS measurements were validated using SLRS-4 frozen river-water reference material for trace metals (National Research Council Canada); and the results (86±7 pg/g, 700±20 pg/g, and 1,810±80 pg/g for Pb, As, and Cu, respectively) exhibited good recovery, as compared with certified values (81±1 pg/g, 680±60 pg/g, and 1,680±70 pg/g for Pb, As, and Cu, respectively). The results confirmed the accuracy of this method and should produce reliable results. Check standards were also analyzed throughout the run to monitor long-term drift. Method blanks were run by a series of vials prepared and treated precisely the same way as the sample vials, the only difference being that they contained deionized water instead of snow samples. The average method blank (sampling blank) value was 0.18 ng/g for Na, 0.06 pg/g for Ba, 0.11 pg/g for Pb, 0.08 pg/g for As, and 0.65 pg/g for Cu. The detection limit in this work, defined as three times the standard deviation of measurements of the blank, was 0.20 ng/g for Na, 0.12 ng/g for Ba, 0.17 pg/g for Pb, 0.11 pg/g for As, and 1.3 pg/g for Cu. Major ions (Na+ and SO42−) were analyzed by Dionexion chromatographs with chemical suppression and conductivity detectors. Anion (SO42−) was measured by use of an AS-11 column, 400-μL sample loop, and a Dionex reagent-free controller producing a KOH eluent gradient of 1–8 mm. Cation (Na+) was measured by use of a CS-12A column, 500-μL loop, with 25 mm of methanesulfonic acid eluent. The precision of the technique for duplicate measurements of a given sample was 1%–5%. The instrumental detection limit was determined by measuring distilled deionized water, as 8 ppb for SO42− and 2 ppb for Na+. More details can be found elsewhere (Hua et al., 2016 ).
3 Results and discussion 3.1 Concentrations of trace elements in the surface snowThe mean concentrations of Pb, As, and Cu for all the samples are 1.04±1.56 pg/g, 0.39±0.08 pg/g, and 11.2±14.4 pg/g, respectively. The concentrations vary from 0.36 pg/g to 9.59 pg/g for Pb, from 0.18 pg/g to 0.62 pg/g for As, and from 0 pg/g to 69 pg/g for Cu (Supplementary Table S1). The As concentrations show a slightly increasing trend with increasing distance from the coast inland, and no apparent trend is found for the concentrations of Pb and Cu. The maximum As concentration is observed near Dome A, while the maximum Cu concentration is in the coastal zone. High Pb concentrations occur in both the coastal zone and inland (Figure 2). When compared with previous records at other Antarctic sites (Table 1), the concentrations for Pb and As in our study area are the lowest observed. Detailed information is discussed later.
|
|
Table 1 Summary of Pb, As, and Cu concentrations in this work and other major research in Antarctica |
Natural sources of trace elements in the atmosphere are predominantly from rock and soil dust, sea-salt spray, and volcanic emissions (Nriagu, 1989). Ba and Na are used as the referential elements to evaluate the contributions from crustal dust and sea-salt (Vallelonga et al., 2002 ; Van de Velde et al., 2005 ; Hong et al., 2012 ; Chang et al., 2016 ). The enrichment factors (EF) and relative contributions of crustal dust and sea-salt have been calculated by the following equations:
| $\begin{align} & EFc\left( X \right) = {\left[ {X/{\rm Ba}} \right]_{\rm {sample}}}/{\left[ {X/{\rm Ba}} \right]_{\rm {crust}}} \\[3pt] & Xc = {{\rm Ba}_{\rm {sample}}} \times {\left[ {X/{\rm Ba}} \right]_{\rm {crust}}}\\[3pt] & EFo\left( X \right) = {\left[ {X/{\rm Na}} \right]_{\rm {sample}}}/{\left[ {X/{\rm Na}} \right]_{\rm {ocean}\;\,\,{\rm {water}}}} \\[3pt]& Xo = {{\rm Na}_{\rm {sample}}} \times {\left[ {X/{\rm Na}} \right]_{\rm {ocean\;\,\, water}}} \\[3pt] \end{align} $ | (1) |
where X = Pb, As, or Cu; Xc and Xo is the fraction of the crustal and oceanic origin of the element, respectively; [X/Ba]crust is the mean metal/Ba ratio in the upper crust (Wedepohl, 1995); and [X/Na]ocean water is the average composition of ocean water (Lide, 2005). EF values lower than 10 usually indicate predominant input from natural mineral dust or sea-salt, while EF values significantly larger than 10 often suggest significant contributions from other sources (Duce et al., 1975 ). The EF values of Pb, As, and Cu in our snow samples (Table 2) suggest that they are dominated by sources other than crustal dust and marine source. This result agrees well with previous work, including Coats Land (Planchon et al., 2002b ), East Queen Maud Land (Hong et al., 2012 ; Chang et al., 2016 ), Dome A (Chang et al., 2016 ; Hua et al., 2016 ), Victoria Land (Grotti et al., 2011 ), WAIS Divide (Koffman et al., 2014 ; Schwanck et al., 2016 ), and ITASE (Dixon et al., 2013 ). Furthermore, the average contributions of both crustal dust and sea-salt are less than 6% for Pb, As, and Cu (Table 2).
|
|
Table 2 Crustal and oceanic enrichment factors and estimated percentages from various natural contributions of trace elements for samples |
The contribution from volcanic emissions in surface snow is estimated from the concentration of non-sea-salt sulfate (nss-SO42−) in each sample and global mean volcanic quiescent degassing values of metal/S ratios (Hinkley et al., 1999 ). We assume that 10%–15% of nss-SO42− originated from volcanoes (Boutron and Patterson, 1986). Although the volcanic contribution is highly variable compared to the crustal-dust and sea-salt sources, the mean percentages of volcanic contribution are also low for Pb (6%–9%), As (9%–14%), and Cu (3%–5%). It is worthwhile to point out that such estimates are fraught with great uncertainty due to the large variation in the published metal/S ratios in volcanic emissions (Patterson and Settle, 1987; Nriagu, 1989; Nho et al., 1996 ).
After excluding the natural fractions tentatively estimated above, the remaining fractions are considered as anthropogenic contributions. It appears that the majority of Pb (>85%), As (>82%), and Cu (>94%) is derived from anthropogenic pollution, such as the combustion of coal, oil, and gasoline; the industry of metal mining and smelting; and local human activity (Pacyna and Pacyna, 2001; Planchon et al., 2002b ; Hur et al., 2007 ; Bargagli, 2008; Thamban and Thakur, 2013; Chang et al., 2016 ).
3.3 Spatial variation of trace-element concentrations along the routeSnow-chemistry concentrations are likely to be influenced by distance from the coast, elevation, topography features, and snow-accumulation rate (Bertler et al., 2005 ; Mahalinganathan et al., 2012 ). According to previous studies (Ren et al., 2001 ; Ma et al., 2010 ; Ding et al., 2011 ), the transect is divided into the coastal area, the intermediate area, and the inland plateau area by the cutoff points at about 200-km (2,000 m a.s.l.) and 800-km (3,000 m a.s.l.) distance to the coast, respectively.
|
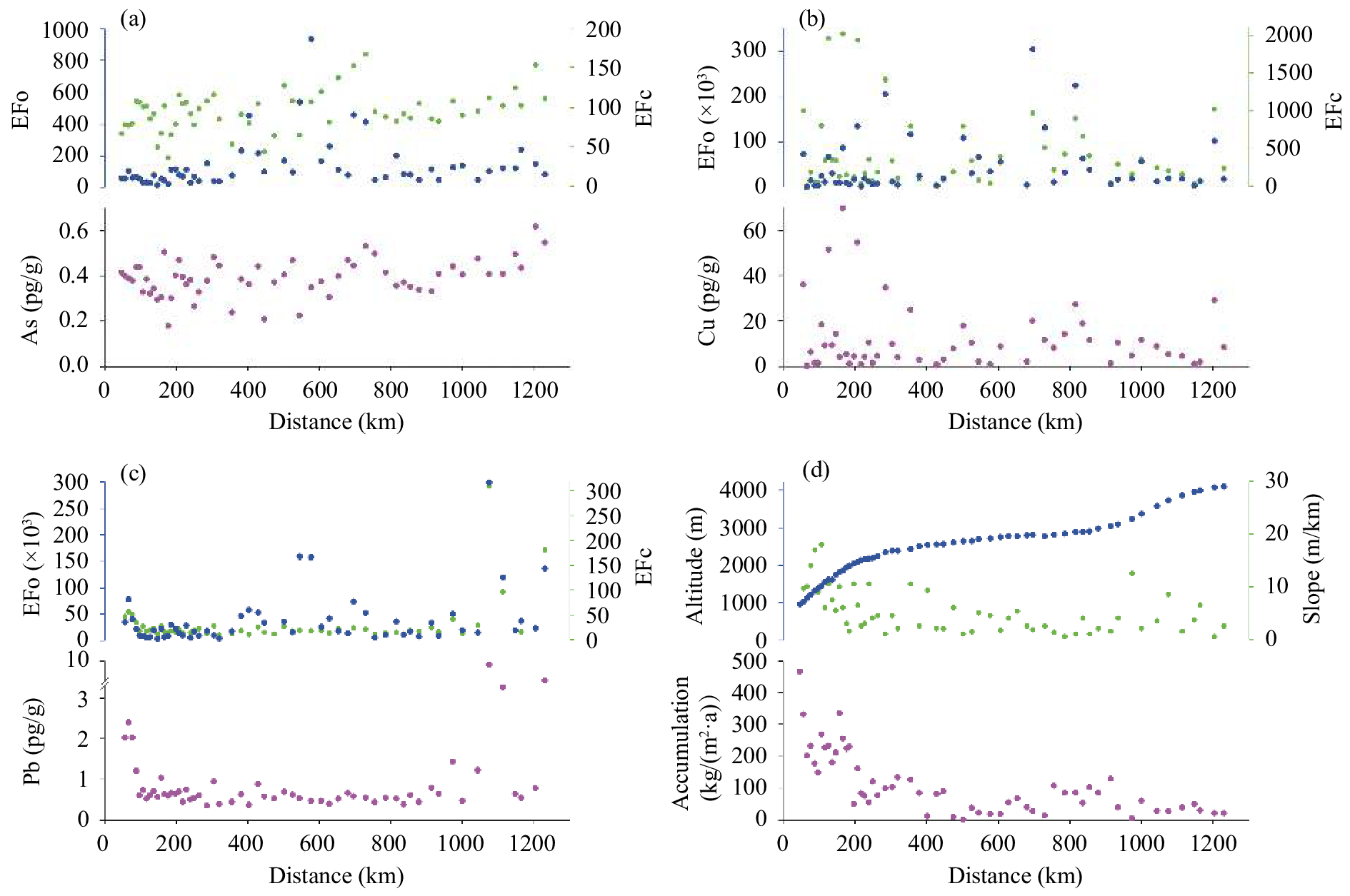
Figure 2 Spatial distributions of Pb, As, and Cu concentrations and enrichment factors (EF) along the transect from the Zhongshan Station to Dome A (a, b, c), together with the physical parameters (altitude, accumulation, and slope) of the transect (d) |
In the coastal area, the concentration of Pb exponentially decreases (y=2.47e−0.17x, R2=0.75, p<0.05) with distance to the coast (from 2.40 pg/g to 0.45 pg/g) (Figure 2c). Based on the source discrimination of the chemical composition of aerosol over the Zhongshan Station (Huang et al., 2003 ), it is reasonable for us to believe that Pb in this region was mainly emitted from local anthropogenic pollution derived from logistic activities or goods transportation, and then transported inland by marine air masses through lower-level advection, which was enhanced by cyclonic systems off the Antarctic Coast. The concentrations of As show a similar but weak negative relationship with distance from coast (Figure 2a), suggesting that As may be less affected by local human activities. There are several peak concentrations of Cu, with no apparent variation tendency along the transect (Figure 2b); and the high concentration of Cu (average 17.9 pg/g) occurred in the coastal area. This finding indicates that Cu in this region also possibly originated from local sources.
In the intermediate area, the Pb, As, and Cu concentrations did not show an apparent spatial trend. However, their concentrations and enrichment factors displayed high variability (Figure 2). The marine air mass from the coast, driven by cyclonic activities, and the continential air mass from the inland plateau, driven by katabatic winds, mixed in the intermediate area. Postdepositional effects such as snow redistribution, wind-driven blowing, and ablation are also remarkable in this area (Ding et al., 2011 ). Both cases can result in the high variabilities of concentrations and enrichment factors of Pb, As, and Cu. The correlation coefficients between the concentrations of Pb and As vs. slope are −0.43 (p<0.05) and −0.48 (p<0.05), respectively, implying that the concentrations of Pb and As may also be influenced by topography in this area.
On the inland plateau, an increasing trend of As concentrations is found with the increase of distance from the coast; and As shows the highest mean concentration (0.43 pg/g); (Figure 2a), which may be attributed to the two unique deposition types (dry deposition and clear-sky precipitation). Dry deposition is the most important sink mechanism on the Antarctic plateau (Wolff et al., 1998 ; Fischer et al., 2007 ), where the accumulation rate is low (Hou et al., 2007 ). The concentrations of As are significantly correlated with the accumulation rate (r=−0.69, p<0.05). The clear-sky precipitation types include "diamond dust" (plates, pyramids, hollow and solid columns) and crystal aggregates (Hou et al., 2007 ; Santachiara et al., 2016 ). This pattern is the result of water-vapor desublimation and is easy for the snow crystals adsorbing impurities from the atmosphere due to its high specific surface area. Both deposition types are beneficial to As enrichment. Besides, another important reason may also account for the observed phenomenon. Based on our previous work on trace elements recorded from a snow pit at Dome A, the result revealed that the major source for As was the pollutants emission from nonferrous metal smelting in South America, especially in Chile. The particles originating from South America deposited on the inland area with a greater proportion (60%) than that of the rest of the area (50%) in terms of Princess Elizabeth Land, according to the general climate model results presented by Albani et al. (2012) and Li et al. (2008) .
Pb also shows a significantly increasing trend from the coast inland. The two unique deposition types aforementioned for As may also apply for Pb although the concentrations and enrichment factors of Pb exhibit high variations, similar to what was observed in the coastal area (Figure 2c). The finding indicates anthropogenic activity is also a significant source responsible for the Pb around stations built inland (Dixon et al., 2013 ; Grotti et al., 2015 ). Our observation also gives support for the existance of polar stratospheric precipitation. The present investigations have observed a poleward air flow derived from the midlatitude high troposphere. It transports water vapor, gases, and aerosols to Antarctica by long-range Brewer-Dobson circulation through the stratosphere and then sinks, replacing the air flowing with the katabatic winds in the Antarctic Plateau (Bargagli, 2008; Stohl and Sodemann, 2010). The Dome A region is the major atmospheric subsidence area; aerosols with fine particle may be enriched here through long-range transport.
Cu does not show a spatial variation similar to that of Pb and As (Figure 2b). It is suggested that wet deposition is a major way for Cu because a significantly positive correlation exists between Cu concentrations and accumulation rate (Table 3). Moreover, the particle size of Cu is coarser than that of Pb and As (Merian et al., 2004 ). Cu is not as likely affected by human activities from the inland station as is Pb. Discharge of daily wastewater and a stack of solid trash makes pollutant elements like Cr, Pb, Cu, and Al enter into soils and snow (Wang et al., 2010 ); however, these activities are scarce inland.
We further performed multiple linear regressions to evaluate the contribution of geophysical factors (i.e., distance from the coast inland, elevation, slope, and accumulation rate) to concentrations of trace elements, with the result of R2=0.22, 0.16, and 0.12 for Pb, As, and Cu, respectively. This result demonstrates that the geophysical features account for only a minority of their variations. The aforementioned factors such as source, transportation pathway, and deposition pattern may play a more vital role for the spatial variation of snow chemistry.
|
|
Table 3 Correlation coefficients of trace elements in the surface-snow samples and their geophysical parameters |
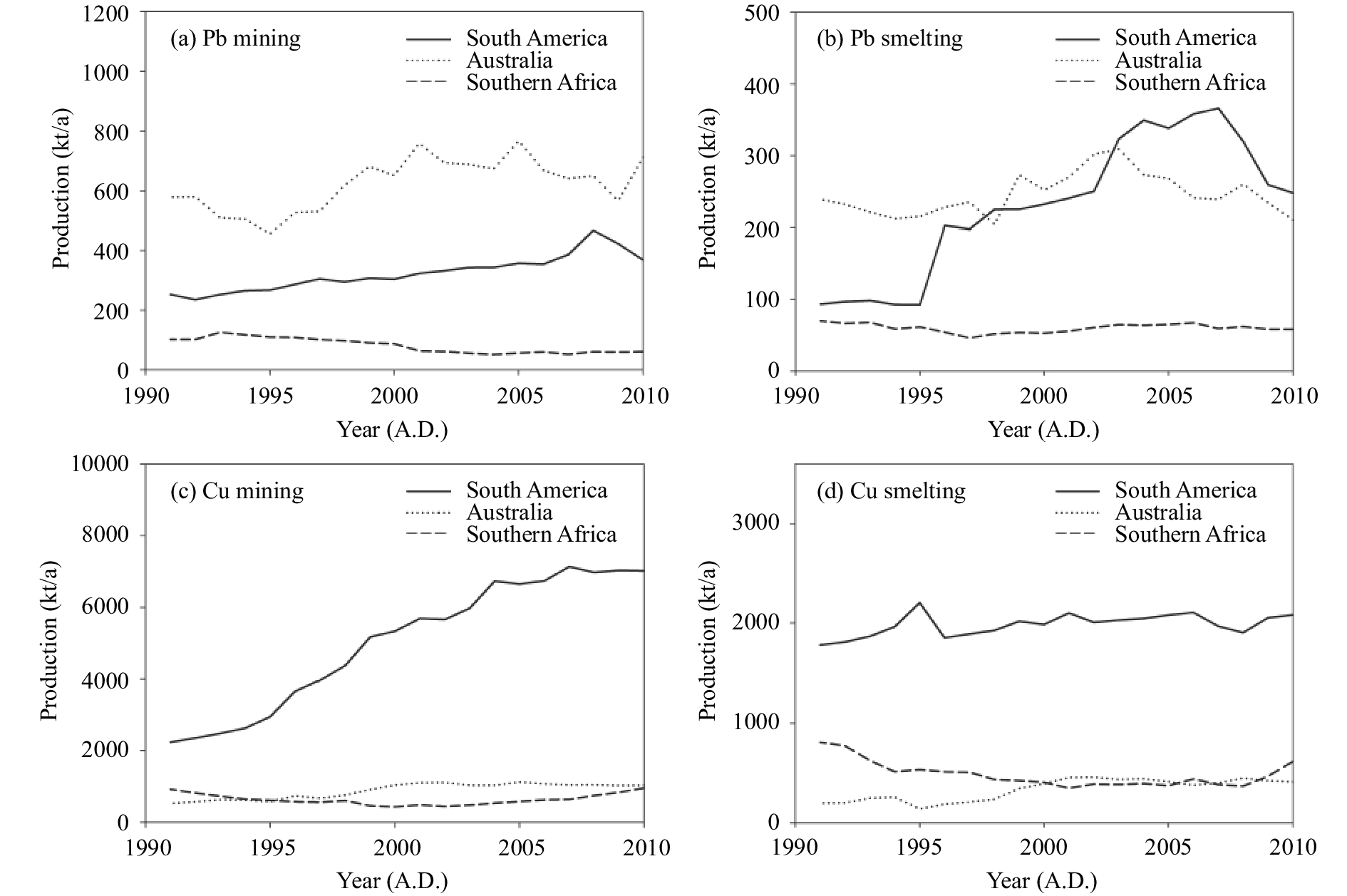
To decipher sources of trace elements and their transport mechanisms, we compared our results with previous work over the entire continent of Antarctica (Table 1 and Figure 3). The mean concentration of As along the traverse from the Zhongshan Station to Dome A is lower than that of other three traverses (i.e., S16–Dome F by Ikegawa et al., 1999 ; ITASE 02 and ITASE 03 by Dixon et al., 2013 ). However, the result does not necessarily indicate that the deposition of As along the traverse from the Zhongshan Station to Dome A is less than along the other three traverses, due to their different sampling time (1993, 2002, 2003, and 2009, respectively); and the data in Ikegawa et al. (1999) are unreliable due to possible pollution (Lee et al., 2008 ). A consistent variation trend was found between the concentrations of As and the production of copper smelting in South America, especially in Chile (Hong et al., 2012 ; Hua et al., 2016 ; Schwanck et al., 2016 ). Although the production of copper smelting in South America has shown no apparent change during the last 20 years (Figure 4), the concentrations of As decreased continually due to strict emission regulations in nonferrous metal smelting (Hua et al., 2016 ; Schwanck et al., 2016 ). Therefore, the later the sampling, the lower the As concentration. The As concentrations show a significant gradual decreasing trend along the direction from Dronning Maud Land (DML), Princess Elizabeth Land (PEL), and Victoria Land (VL) to WAIS Divide (WD) (Figure 3). This spatial variation pattern coincides fairly well with the modeling results of the transport pathway of particles from South America to Antarctica (Li et al., 2008 ; Albani et al., 2012 ). Copper smelting in South America accounts for 70% of the total production in the Southern Hemisphere (Figure 4). Therefore, the spatial distribution of As further confirms that As pollution in Antarctica was mainly attributable to the industrial copper activity of South America.

|
Figure 3 Spatial distribution of As, Pb, and Cu concentrations across Antarctica. Solid circles stand for surface-snow data and solid triangles for snow-pit data. DML, PEL, VL, and WD stand for Dronning Maud Land, Princess Elizabeth Land, Victoria Land, and WAIS Divide, respectively. The relative contribution (fraction) from the three Southern Hemispheric sources (SH) to particle deposition in high-latitude SH under the modern-day environment is shown in (d) (Li et al., 2008 ). Only contributions larger than 30% are plotted, with the red, blue, and green shadings representing the contributions from South America, Southern Africa, and Australia, respectively |
|
Figure 4 Historical changes in nonferrous metal (Pb and Cu) production in South America, Australia, and Southern Africa during the last 20 years. Data are from the British Geological Survey's World Mineral Statistics and Mineral Yearbook (US Bureau of Mines) |
The mean concentration of Pb along the traverse from the Zhongshan Station to Dome A is also lower than that of other traverses (Table 1). It is suggested that Pb concentrations decreased by a factor of 4 during the last 20 years (McConnell et al., 2014 ; Chang et al., 2016 ). Thus the difference between our results and those of previous studies may be partly caused by sampling times. Based on the variations of concentrations and isotopic signatures of Pb in the Antarctic ice cores and snow pits, it is suggested that three major sources (i.e., copper smelting in South America, lead mining from Broken Hills in Australia, and leaded petrol usage) contributed to Pb deposition on Antarctic snow and ice during recent decades (Wolff and Suttie, 1994; Vallelonga et al., 2002 ; Burn-Nunes et al., 2011 ; McConnell et al., 2014 ; Chang et al., 2016 ). The multiple sources and fine particle size of Pb (Merian et al., 2004 ) allow it to be mixed well in the Antarctic atmosphere. Li et al. (2008) and Albani et al. (2012) suggested that Pb emission from copper mining and smelting in South America mainly reached the Atlantic and Indian Ocean sector of Antarctica, while Pb emission from Broken Hill Pb mining in Australia, the largest area of Pb production in the Southern Hemisphere (Figure 4), reached only Victoria Land and West Antarctica. Although some countries in Southern Africa, such as Zaire and Zambia, also perform nonferrous metal smelting and mining activities, their production is much less than that of South America and Australia (Figure 4). Therefore, the African contribution should be neglected.
To the contrary, the mean concentration of Cu along the traverse from the Zhongshan Station to Dome A is much higher than that of S16–Dome F (Table 1). It is suggested that the copper–nickel industry was the largest contributor of Cu in the atmosphere (Nriagu and Pacyna, 1988; Pacyna and Pacyna, 2001). Wolff et al. (1999) investigated an Antarctic snow pit, covering the period from 1923 to 1986, and suggested a link between Cu concentrations in Antarctic snow and copper mining in South America. The production of Cu in South America over the past decades has been increasing (Figure 4). Chile, the largest copper-mining country in South America (about 90%), began to carry out environmental regulations governing toxic-metals emissions from the copper industry since the 1990s. This policy mainly affected arsenic emission due to a special law DS-165 (from 1999) regulating arsenic emissions into the atmosphere (Schwanck et al., 2016 ), but it had little effect on copper emission (Caldentey and Mondschein, 2003). So the much higher mean concentration of Cu in our samples than in S16–Dome F may be caused by increasing production of copper mining in the Southern Hemisphere. The concentrations of Cu in West Antarctica are much higher than those of East Antarctica (Figure 3), except Asuka Station and Terra Nova Bay, which were obviously influenced by local sources (Suttie and Wolff, 1993), The possible reason is that the transport of air masses through the low-level atmosphere is more frequent from Australia than from South America (Li et al., 2008 ; Krinner et al., 2010 ), and Cu production in Australia is only one-sixth of that in South America (Figure 4). Because results of Cu in Antarctica are currently scarce, more work is needed to examine further the spatial pattern of Cu over Antarctica.
4 ConclusionsThis study presents the spatial distribution of Pb, As, and Cu concentrations in the surface snow along the route from the Zhongshan Station to Dome A in East Antarctica. In the coastal area, Pb and Cu show extremely high concentrations due to local human activities, while As keeps at a low concentration. In the intermediate area, the concentrations and enrichment factors of all three trace elements show high variability due to the complicated characteristics of climate and environment. On the inland plateau, the high concentrations of As and Pb are induced by high deposition efficiency, polar stratospheric precipitation, and the different fraction deposition to East Antarctica. These results suggest that source, transport pathway, and deposition pattern are more vital than geographic features (i.e., distance from the coast, altitude, and slope) for controlling the spatial distribution of Pb, As, and Cu in Antarctica. It is also suggested that Pb, As, and Cu in the Antarctic surface snow are primarily derived from anthropogenic pollutants. Given the wide area of Antarctica, more data are necessary for a better understanding of the spatial distribution of the trace metals, together with their possible sources, transport, and depositional processes.
Acknowledgments:The authors would like to thank the Chinese 26th Antarctic Expedition. In addition, special thanks are given to Mr. JinHai Yu and Mr. Xiang Zou for their useful comments and suggestions. This work was supported by the National Natural Science Foundation of China (41330526).
Albani S, Mahowald NM, Delmonte B, et al.. 2012. Comparing modeled and observed changes in mineral dust transport and deposition to Antarctica between the last glacial maximum and current climates. Climate Dynamics, 38(9–10): 1731-1755. DOI:10.1007/s00382-011-1139-5 |
Bargagli R. 2008. Environmental contamination in Antarctic ecosystems. Science of the Total Environment, 400(1–3): 212-226. DOI:10.1016/j.scitotenv.2008.06.062 |
Bertler N, Mayewski PA, Aristarain A, et al.. 2005. Snow chemistry across Antarctica. Annals of Glaciology, 41: 167-179. DOI:10.3189/172756405781813320 |
Boutron CF, Patterson CC. 1986. Lead concentration changes in Antarctic ice during the Wisconsin/Holocene transition. Nature, 323(6085): 222-225. DOI:10.1038/323222a0 |
Boutron CF, Patterson CC. 1987. Relative levels of natural and anthropogenic lead in recent Antarctic snow. Journal of Geophysical Research: Atmospheres, 92(D7): 8454-8464. DOI:10.1029/jd092id07p08454 |
Burn-Nunes LJ, Vallelonga P, Loss RD, et al.. 2011. Seasonal variability in the input of lead, barium and indium to Law Dome, Antarctica. Geochimica et Cosmochimica Acta, 75(1): 1-20. DOI:10.1016/j.gca.2010.09.037 |
Caldentey R, Mondschein S. 2003. Policy model for pollution control in the copper industry, including a model for the sulfuric acid market. Operations Research, 51(1): 1-16. DOI:10.1287/opre.51.1.1.12797 |
Chang C, Han C, Han Y, et al.. 2016. Persistent Pb pollution in central East Antarctic snow: a retrospective assessment of sources and control policy implications. Environmental Science & Technology, 50(22): 12138-12145. DOI:10.1021/acs.est.6b03209 |
Chen WQ, Graedel TE. 2012. Anthropogenic cycles of the elements: a critical review. Environmental Science & Technology, 46(16): 8574-8586. DOI:10.1021/es3010333 |
Ding MH, Xiao CD, Li YS, et al.. 2011. Spatial variability of surface mass balance along a traverse route from Zhongshan station to Dome A, Antarctica. Journal of Glaciology, 57(204): 658-666. DOI:10.3189/002214311797409820 |
Dixon DA, Mayewski PA, Korotkikh E, et al.. 2013. Variations in snow and firn chemistry along US ITASE traverses and the effect of surface glazing. The Cryosphere, 7(2): 515-535. DOI:10.5194/tc-7-515-2013 |
Duce RA, Hoffman GL, Zoller WH. 1975. Atmospheric trace metals at remote northern and southern hemisphere sites: pollution or natural?. Science, 187(4171): 59-61. DOI:10.1126/science.187.4171.59 |
Fischer H, Siggaard Andersen ML, Ruth U, et al.. 2007. Glacial/interglacial changes in mineral dust and sea-salt records in polar ice cores: Sources, transport, and deposition. Reviews of Geophysics, 45(1): RG1002. DOI:10.1029/2005rg000192 |
Grotti M, Soggia F, Ardini F, et al.. 2011. Major and trace element partitioning between dissolved and particulate phases in Antarctic surface snow. Journal of Environmental Monitoring, 13(9): 2511-2520. DOI:10.1039/c1em10215j |
Grotti M, Soggia F, Ardini F, et al.. 2015. Year-round record of dissolved and particulate metals in surface snow at Dome Concordia (East Antarctica). Chemosphere, 138: 916-923. DOI:10.1016/j.chemosphere.2014.10.094 |
Hinkley TK, Lamothe PJ, Wilson SA, et al.. 1999. Metal emissions from Kilauea, and a suggested revision of the estimated worldwide metal output by quiescent degassing of volcanoes. Earth & Planetary Science Letters, 170(3): 315-325. DOI:10.1016/s0012-821x(99)00103-x |
Hong S, Lluberas A, Rodriguez F. 2000. A clean protocol for determining ultralow heavy metal concentrations: its application to the analysis of Pb, Cd, Cu, Zn and Mn in Antarctic snow. Korean Journal of Polar Research, 11(1): 35-47. |
Hong S, Soyol-Erdene TO, Hwang HJ, et al.. 2012. Evidence of global-scale As, Mo, Sb, and Tl atmospheric pollution in the Antarctic Snow. Environmental Science & Technology, 46(21): 11550-11557. DOI:10.1021/es303086c |
Hou SG, Li YS, Xiao CD, et al.. 2007. Recent accumulation rate at Dome A, Antarctica. Chinese Science Bulletin, 52(3): 428-431. DOI:10.1007/s11434-007-0041-3 |
Hua R, Hou SG, Li YS, et al.. 2016. Arsenic record from a 3 m snow pit at Dome Argus, Antarctica. Antarctic Science, 28(4): 305-312. DOI:10.1017/s0954102016000092 |
Huang ZQ, Ji WD, Yang XL, et al.. 2003. The chemical composition of aerosol over Zhongshan Station in Antarctica and its sources discrimination. Journal of Oceanography in Taiwan Strait, 22(3): 334-346. DOI:10.3969/j.issn.1000-8160.2003.03.011 |
Hur SD, Xiao C, Hong S, et al.. 2007. Seasonal patterns of heavy metal deposition to the snow on Lambert Glacier Basin, East Antarctica. Atmospheric Environment, 41(38): 8567-8578. DOI:10.1016/j.atmosenv.2007.07.012 |
Ikegawa M, Kimura M, Honda K, et al.. 1997. Springtime peaks of trace metals in Antarctic snow. Environmental Health Perspectives, 105(6): 654-659. DOI:10.2307/3433612 |
Ikegawa M, Kimura M, Honda K, et al.. 1999. Geographical variations of major and trace elements in East Antarctica. Atmospheric Environment, 33(9): 1457-1467. DOI:10.1016/s1352-2310(98)00243-x |
Järup L. 2003. Hazards of heavy metal contamination. British Medical Bulletin, 68(1): 167-182. DOI:10.1093/bmb/ldg032 |
Koffman BG, Handley MJ, Osterberg EC, et al.. 2014. Dependence of ice-core relative trace-element concentration on acidification. Journal of Glaciology, 60(219): 103-112. DOI:10.3189/2014JoG13J137 |
Krinner G, Petit JR, Delmonte B. 2010. Altitude of atmospheric tracer transport towards Antarctica inpresent and glacial climate. Quaternary Science Reviews, 29(1–2): 274-284. DOI:10.1016/j.quascirev.2009.06.020 |
Lee K, Do Hur S, Hou SG, et al.. 2008. Atmospheric pollution for trace elements in the remote high-altitude atmosphere in central Asia as recorded in snow from Mt. Qomolangma (Everest) of the Himalayas. Science of the Total Environment, 404(1): 171-181. DOI:10.1016/j.scitotenv.2008.06.022 |
Li FY, Ginoux P, Ramaswamy V. 2008. Distribution, transport, and deposition of mineral dust in the southern ocean and Antarctica: contribution of major sources. Journal of Geophysical Research: Atmospheres, 113(D10): D10207. DOI:10.1029/2007jd009190 |
Lide DR, 2005. Abundance of Elements in the Earth's crust and in the sea. In: CRC Handbook of Chemistry and Physics. Boca Raton: CRC Press, pp. 14–17.
|
Liu YP, Hou SG, Hong SM, et al.. 2011. High-resolution trace element records of an ice core from the eastern Tien Shan, central Asia, since 1953 AD. Journal of Geophysical Research: Atmospheres, 116(D12): D12307. DOI:10.1029/2010jd015191 |
Mahalinganathan K, Thamban M, Laluraj CM, et al.. 2012. Relation between surface topography and sea-salt snow chemistry from Princess Elizabeth Land, East Antarctica. The Cryosphere, 6(2): 505-515. DOI:10.5194/tcd-5-2967-2011 |
Ma YF, Bian LE, Xiao CD, et al.. 2010. Near surface climate of the traverse route from Zhongshan Station to Dome A, East Antarctica. Antarctic Science, 22(4): 443-459. DOI:10.1017/s0954102010000209 |
McConnell JR, Maselli OJ, Sigl M, et al.. 2014. Antarctic-wide array of high-resolution ice core records reveals pervasive lead pollution began in 1889 and persists today. Scientific Reports, 4: 5848. DOI:10.1038/srep05848 |
Merian E, Anke M, Ihnat M, et al., 2004. Elements and their compounds in the environment: occurrence, analysis and biological relevance. Weinheim: Wiley. DOI: 10.1002/9783527619634.
|
Murozumi M, Chow TJ, Patterson C. 1969. Chemical concentrations of pollutant lead aerosols, terrestrial dusts and sea salts in Greenland and Antarctic snow strata. Geochimica et Cosmochimica Acta, 33(10): 1247-1294. DOI:10.1016/0016-7037(69)90045-3 |
Nho EY, Le Cloarec MF, Ardouin B, et al.. 1996. Source strength assessment of volcanic trace elements emitted from the Indonesian arc. Journal of Volcanology and Geothermal Research, 74(1–2): 121-129. DOI:10.1016/S0377-0273(96)00051-0 |
Nriagu JO, Davidson CI, 1986. Toxic Metals in the Atmosphere. New York: John Wiley and Sons, pp. 635.
|
Nriagu JO, Pacyna JM. 1988. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333(6169): 134-139. DOI:10.1038/333134a0 |
Nriagu JO. 1989. A global assessment of natural sources of atmospheric trace metals. Nature, 338(6210): 47-49. DOI:10.1038/338047a0 |
Osterberg EC, Handley MJ, Sneed SB, et al.. 2006. Continuous ice core melter system with discrete sampling for major ion, trace element, and stable isotope analyses. Environmental Science & Technology, 40(10): 3355-3361. DOI:10.1021/es052536w |
Pacyna JM, Pacyna EG. 2001. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environmental Reviews, 9(4): 269-298. DOI:10.1139/er-9-4-269 |
Patterson CC, Settle DM. 1987. Magnitude of lead flux to the atmosphere from volcanoes. Geochimica et Cosmochimica Acta, 51(3): 675-681. DOI:10.1016/0016-7037(87)90078-0 |
Planchon FAM, Boutron CF, Barbante C, et al.. 2002a. Changes in heavy metals in Antarctic snow from Coats Land since the mid-19th to the late-20th century. Earth and Planetary Science Letters, 200(1–2): 207-222. DOI:10.1016/s0012-821x(02)00612-x |
Planchon FAM, Boutron CF, Barbante C, et al.. 2002b. Short-term variations in the occurrence of heavy metals in Antarctic snow from Coats Land since the 1920s. Science of the Total Environment, 300(1–3): 129-142. DOI:10.1016/s0048-9697(02)00277-2 |
Potocki M, Mayewski PA, Kurbatov AV, et al.. 2016. Recent increase in Antarctic Peninsula ice core uranium concentrations. Atmospheric Environment, 140: 381-385. DOI:10.1016/j.atmosenv.2016.06.010 |
Ren JW, Qin DH, Xiao CD. 2001. Preliminary results of the inland expeditions along a transect from the Zhongshan Station to Dome A, East Antarctica. Journal of Glaciology and Geocryology, 23(1): 51-56. |
Santachiara G, Belosi F, Prodi F. 2016. Ice crystal precipitation at Dome C site (East Antarctica). Atmospheric Research, 167: 108-117. DOI:10.1016/j.atmosres.2015.08.006 |
Schwanck F, Simões JC, Handley M, et al.. 2016. Anomalously high arsenic concentration in a west Antarctic ice core and its relationship to copper mining in Chile. Atmospheric Environment, 125: 257-264. DOI:10.1016/j.atmosenv.2015.11.027 |
Stohl A, Sodemann H. 2010. Characteristics of atmospheric transport into the Antarctic troposphere. Journal of Geophysical Research: Atmospheres, 115(D2): D02305. DOI:10.1029/2009jd012536 |
Suttie ED, Wolff EW. 1993. The local deposition of heavy metal emissions from point sources in Antarctica. Atmospheric Environment. Part A. General Topics, 27(12): 1833-1841. DOI:10.1016/0960-1686(93)90288-a |
Thamban M, Thakur RC. 2013. Trace metal concentrations of surface snow from Ingrid Christensen coast, East Antarctica—spatial variability and possible anthropogenic contributions. Environmental Monitoring and Assessment, 185(4): 2961-2975. DOI:10.1007/s10661-012-2764-0 |
Vallelonga P, Van de Velde K, Candelone JP, et al.. 2002. The lead pollution history of Law Dome, Antarctica, from isotopic measurements on ice cores: 1500 AD to 1989 AD. Earth and Planetary Science Letters, 204(1–2): 291-306. DOI:10.1016/s0012-821x(02)00983-4 |
Vallelonga P, Barbante C, Cozzi G, et al.. 2004. Elemental indicators of natural and anthropogenic aerosol inputs to Law Dome, Antarctica. Annals of Glaciology, 39: 169-174. DOI:10.3189/172756404781814483 |
Van de Velde K, Vallelonga P, Candelone JP, et al.. 2005. Pb isotope record over one century in snow from Victoria Land, Antarctica. Earth and Planetary Science Letters, 232(1–2): 95-108. DOI:10.1016/j.jpgl.2005.01.007 |
Wang JJ, Chen LQ, Yang XL, et al.. 2010. Characteristics of metals in the aerosols of Zhongshan station, Antarctica. Advances in Polar Science, 21(1): 46-59. DOI:10.3724/SP.J.1085.2010.00046 |
Wedepohl KH. 1995. The composition of the continental crust. Geochimica et Cosmochimica Acta, 59(7): 1217-1232. DOI:10.1016/0016-7037(95)00038-2 |
Wolff EW, Suttie ED. 1994. Antarctic snow record of southern hemisphere lead pollution. Geophysical Research Letters, 21(9): 781-784. DOI:10.1029/94gl00656 |
Wolff EW, Hall JS, Mulvaney R, et al.. 1998. Relationship between chemistry of air, fresh snow and firn cores for aerosol species in coastal Antarctica. Journal of Geophysical Research: Atmospheres, 103(D9): 11057-11070. DOI:10.1029/97jd02613 |
Wolff EW, Suttie ED, Peel DA. 1999. Antarctic snow record of cadmium, copper, and zinc content during the twentieth century. Atmospheric Environment, 33(10): 1535-1541. DOI:10.1016/s1352-2310(98)00276-3 |
 2018, Vol. 10
2018, Vol. 10
