Chlorophyll fluorescence-induction kinetics technology is a fast and nondestructive probe to measure the photosynthetic function of leaves (Genty et al., 1989 ; Schreiber et al., 1994 ; Peng et al., 2017 ); compared with the "apparent" gas-exchange index, which better reflects "intrinsic" characteristics. The chlorophyll fluorescence-induction kinetics curve (OJIP) can provide a large amount of information about photosystem II (PSII) and is mainly used to analyze changes in the energy status of electron transport and the PSII reaction center at the donor side and receptor side of plant PSII (Li et al., 2005 ; Said et al., 2013 ). The growth and development (Dai et al., 2004 ), nutritional status (Nie et al., 1999 ), and adversity stress of plants (Zhao and Wang, 2002; Chen et al., 2004 ; Xia et al., 2014 ) can directly or indirectly affect the PSII function of plant leaves. Therefore, changes in the chlorophyll fluorescence-induction kinetics curve (OJIP) under different growth stages and environmental conditions can reflect the photosynthetic physiological conditions of plants; the influence of various external factors on its photosynthetic structure, especially PSII; and the impact of photosynthetic structure on its adaptation mechanism to the environment.
P. euphratica is the oldest tree species in the genus Populus with a fossil record that extends back 3–6 million years (Wang et al., 1995 ). P. euphratica has developed heteromorphic leaves due to long-term adaptation to extreme drought in a continental climate. The lower branches of seedlings and young trees have lanceolate leaves, while adult trees have lanceolate, ovate-orbicular, reniform, or deltoid-ovate (Su et al., 2003 ). In recent years, studies on heteromorphic leaves of P. euphratica have increasingly focused on their anatomical structure, stomatal characteristics, gas-exchange characteristics, and response to environmental factors (Wang et al., 1998 ; Su et al., 2003 ; Qiu et al., 2005 ). Research that relates to the chlorophyll fluorescence feature mainly analyzed basic fluorescence parameters (Wang et al., 2001; Zheng et al., 2006 ), lacking a systematic comparison and discussion of the seasonal changes and characteristics of fluorescence dynamics curves. Chlorophyll fluorescence-induction kinetics curve (OJIP) analysis of P. euphratica can provide a theoretical basis for the primitive photochemical reaction of the PSII reaction for research on the photosynthetic characteristics of P. euphratica heteromorphic leaves. This analysis can reveal the energy-distribution mechanism of P. euphratica heteromorphic leaves.
In this paper, the research area is a natural population of P. euphratica in the Ejina Desert area of China, which is one of three remaining natural P. euphratica forests in the world. Our objectives for P. euphratica are: 1, measure lanceolate and serrate oval leaves using the chlorophyll fluorescence-induction kinetics curve (OJIP); 2, study the seasonal dynamics of the chlorophyll fluorescence-induction kinetics of heteromorphic leaves; 3, reveal different photosynthetic functions and adaptability mechanisms of heteromorphic leaves; 4, provide a theoretical basis for further understanding the growth and development characteristics of heteromorphic leaves, drought-resistance mechanism, and protection of P. euphratica.
2 Materials and methods 2.1 Overview of the research areaOur experiment was performed at the P. euphratica test sample area of Alashan Desert Hydrological Station (Figure 1), Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, which is downstream of Heihe River headwaters, with an altitude of 883.54 m. The area is deeply inland with dry climate, a typical continental climate. Average annual rainfall is 37.9 mm, with an evaporation amount up to 3,700 mm or above (Chang et al., 2006 ); average annual temperature is 8.3 °C, extreme highest temperature is 41.6 °C, extreme lowest temperature is −36.1 °C, average temperature difference is 17.2 °C, and the maximum daily temperature difference is 29.1 °C (Su et al., 2004 ).

|
Figure 1 The locality of research area |
In the experimental field, lanceolate and serrate oval leaves were simultaneously collected from young P. euphratica trees that had straight trunks and lacked pests or disease. The third or fourth leaf on each branch that extended toward the sun was chosen for measurement.
2.3 Research methodsOJIP parameters were measured by a portable chlorophyll fluorescence instrument (OS-30P+) for different types of poplar leaves. The curve was induced by saturated light of 3,500 μmol/(m2·s), and the fluorescence signal record started at 10 μs and ended at 3 s. A dark-adaptation clip was used to keep the leaf in darkness for at least 30 min before measuring; and the instrument was turned on to warm up and set the measurement parameters. The measurement time was set as 9:00–10:00 a.m. on a sunny day during the growth season (from May to September) of 2013. When measuring fluorescence parameters, we chose five replicates of each kind of leaf.
2.4 Data analysisSeasonal characteristics of the chlorophyll fluorescence kinetics for heteromorphic leaves of P. euphratica were analyzed using the statistical analysis software SPSS 13.0 for Windows, and the dynamic curve was drawn using Origin Pro 8.0.
3 Results and analysis 3.1 OJIP seasonal dynamics of heteromorphic leaves in P. euphraticaAccording to the chlorophyll fluorescence-induction kinetics primitive curves for lanceolate and serrate oval leaves of P. euphratica from May to September (Figure 1), we found that the two kinds of heteromorphic leaves are typical OJIP curves. There were varied degrees of differences in the fluorescence signals of each phase between heteromorphic leaves in the same month, and in the same kind of leaves in different months (Figure 2 and Figure 3). The fluorescence signals at phases O, J, and I of lanceolate leaves in May and September were stronger than those of serrate oval leaves, this difference being the most obvious in May. In other months, the fluorescence signals at phases O, J, and I of lanceolate leaves were slightly lower than those of serrate oval leaves. In each month, serrate oval leaves arrived at P phase much earlier than lanceolate leaves; and the fluorescence of the P phase was stronger than that of lanceolate leaves. The fluorescence intensity of serrate oval and lanceolate leaves in May and June was significantly greater than that in July, August, and September.

|
Figure 2 Variation of the fluorescence signals between heteromorphic leaves of P. euphratica from May to September in 2013: (a) May; (b) June; (c) July; (d) August; and (e) September |
|
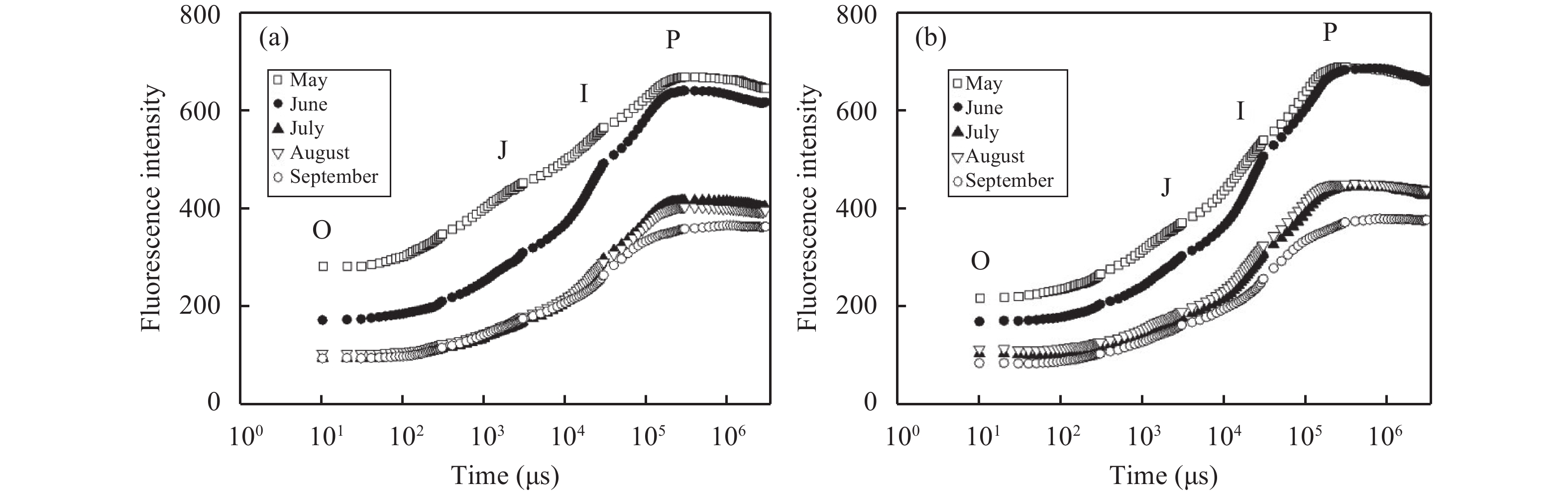
Figure 3 Chlorophyll fluorescence-induction curves of lanceolate (a) and serrate oval leaves (b) of Populus euphratica Oliver in different months |
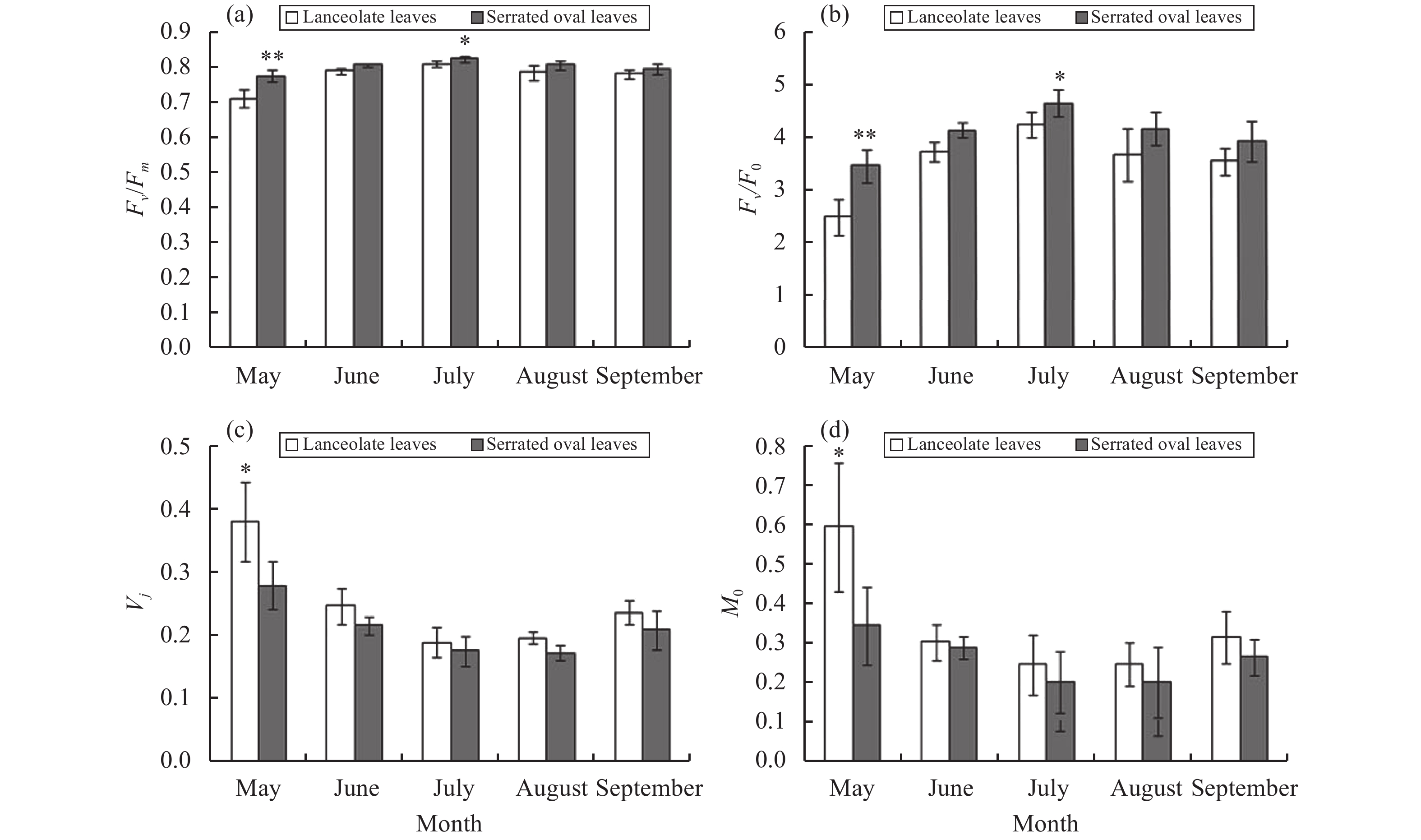
The PSII maximum photochemical efficiency Fv/Fm and PSII potential activity Fv/F0 of serrate oval leaves of P. euphratica were higher than those of lanceolate leaves in May, and the difference was most significant in May and July (Figure 3). Fv/Fm and Fv/F0 of the two kinds of leaves reached maximum values in July. The variable fluorescence at J phase Vj and the relative speed of QA deoxidation (M0) of serrate oval leaves were smaller than those of lanceolate leaves in each month, and the difference was most significant in May (Figure 4). The two kinds of leaves basically had the same trends of seasonal changes in Vj and M0, and they had the lowest Vj and M0 in July.
|
Figure 4 Seasonal variation of fluorescence parameters (a–d) for heteromorphic leaves ofP. euphratica: (a) Fv/Fm, (b) Fv/F0, (c) Vj, and (d) M0 |
During the whole growing season, the number of active reaction centers in a unit cross-sectional area of P. euphratica serrate oval leaves was larger than that of lanceolate leaves. The energy ABS/CS0, which was absorbed by a unit cross-sectional area of P. euphratica heteromorphic leaves, had no significant difference except in May. The energy DI0/CS0, which is used for heat dissipation by a unit cross-sectional area of serrate oval leaves, was lower than that of lanceolate leaves; and the energy ET0/CS0, which is used for electron transport on a unit cross-sectional area of serrate oval leaves, was higher than that of lanceolate leaves. During each month, the energy TR0/CS0, which was captured on a unit cross-sectional area of the two kinds of leaves, did not have a consistently changing trend. During the whole growing season, the mean values of TR0/CS0 for serrate oval and lanceolate lea-ves were 86.7% and 94.3%, respectively (Figure 5).

|
Figure 5 Seasonal variation of energy fluxes per cross section (a–e) for heteromorphic leaves of P. euphratica: (a) RC/CS0; (b) ABS/CS0; (c) DI0/CS0; (d) ET0/CS0; and (e) TR0/CS0 |
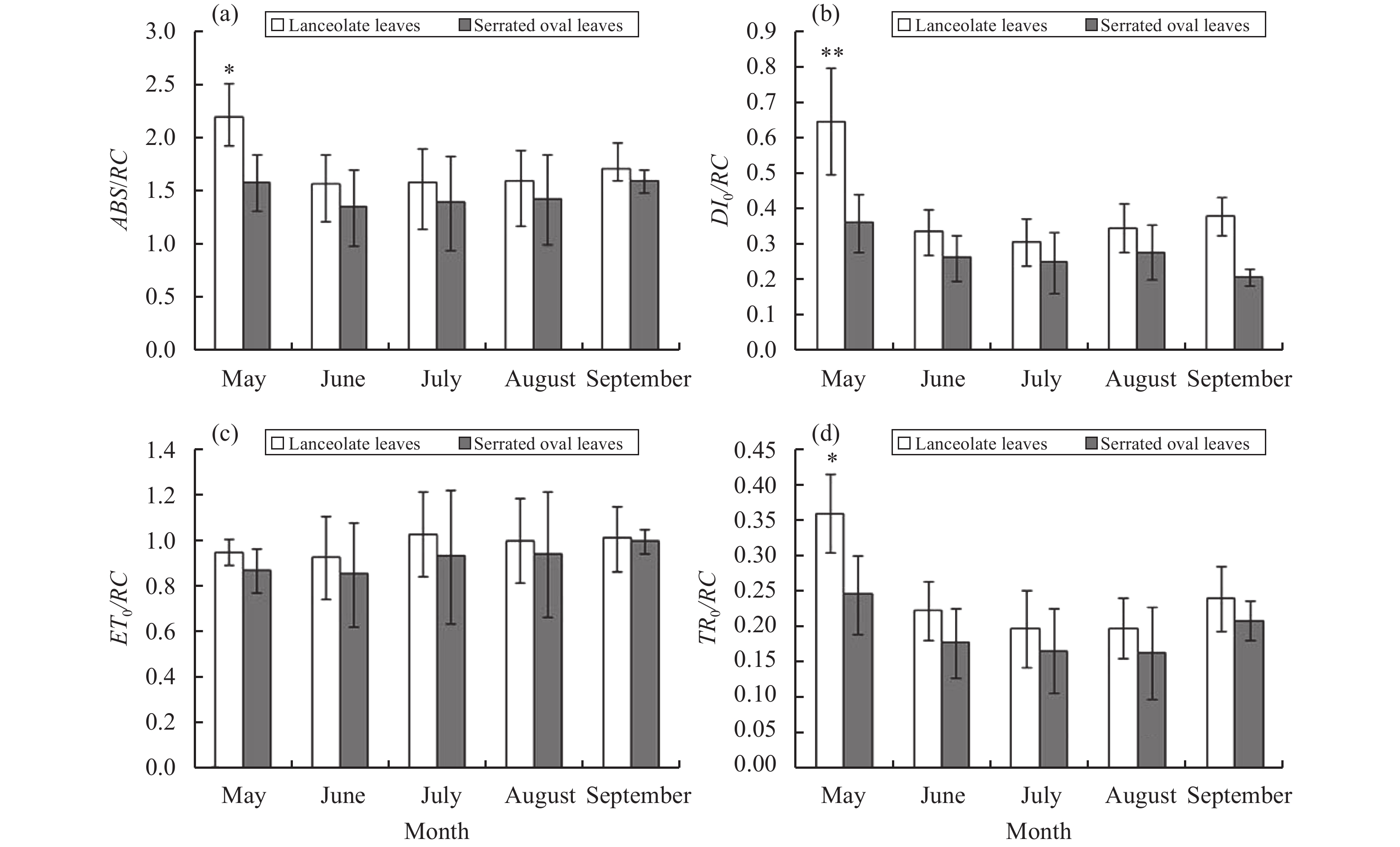
The energy-flow parameters (ABS/RC, ET0/RC, TR0/RC, and DI0/RC) of P. euphratica lanceolate leaves with active reaction centers were larger than those of serrate oval leaves from May to September (Figure 6). The number of reaction centers in a unit cross-sectional area of lanceolate leaves was relatively small. Under the same illumination area and intensity, a single reaction center of a lanceolate leaf received more energy; and its burden increased. Ultimately, the absorbed energy ABS/RC, the captured energy TR0/RC, the energy for electron transport ET0/RC, and the dissipated energy DI0/RC in a unit reaction center of lanceolate leaves were higher than those of serrate oval leaves.
|
Figure 6 Energy-flow parameters of P. euphratica heteromorphic leaves with active reaction centers, from May to September: (a) ABS/RC, (b) ET0/RC, (c) TR0/RC, and (d) DI0/RC |
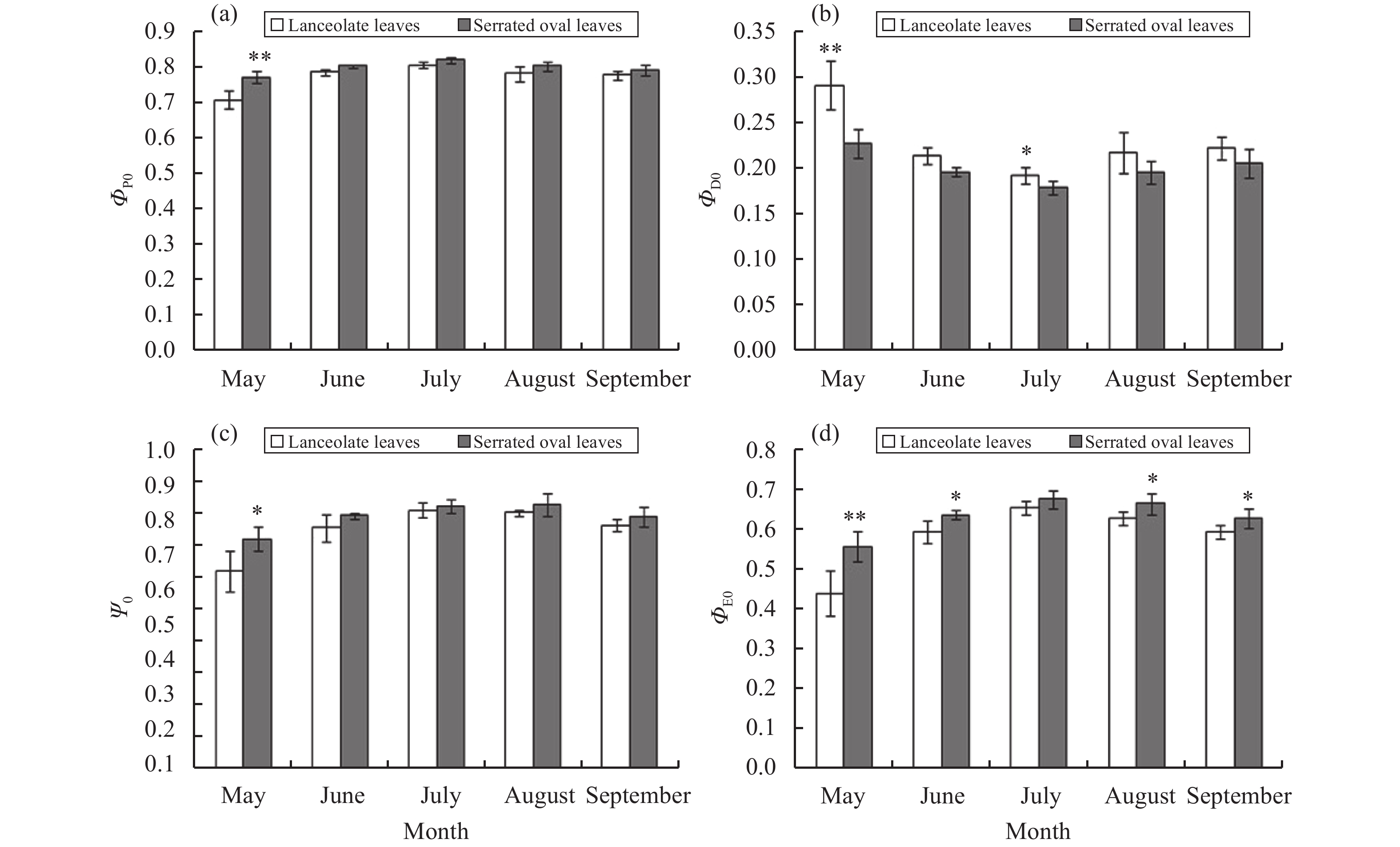
In the energy distribution (Figure 7) of heteromorphic leaves, the maximum quantum yield ΦP0 of the original photochemical reaction of serrate oval leaves was higher than that of lanceolate leaves; the maximum quantum yield ΦD0 of nonphotochemical quenching was lower than that of lanceolate leaves; and the differences were most significant in May and July. The proportion of the light energy captured by P. euphratica serrate oval leaves and the proportion of light energy absorbed for electron transport Ψ0 and ΦE0 are greater than those of lanceolate leaves. The seasonal change trends of ΦP0, Ψ0, and ΦE0 for P. euphratica heteromorphic leaves were basically the same in different months, with a maximum value in July and a minimum in May. The seasonal change trend of ΦD0 is the opposite, with a maximum value in May and a minimum in July.
|
Figure 7 Seasonal variation distribution of energy-flux ratios (a–d) for heteromorphic leaves of P. euphratica: (a) ΦP0, (b) ΦD0, (c) Ψ0, and (d) ΦE0 |
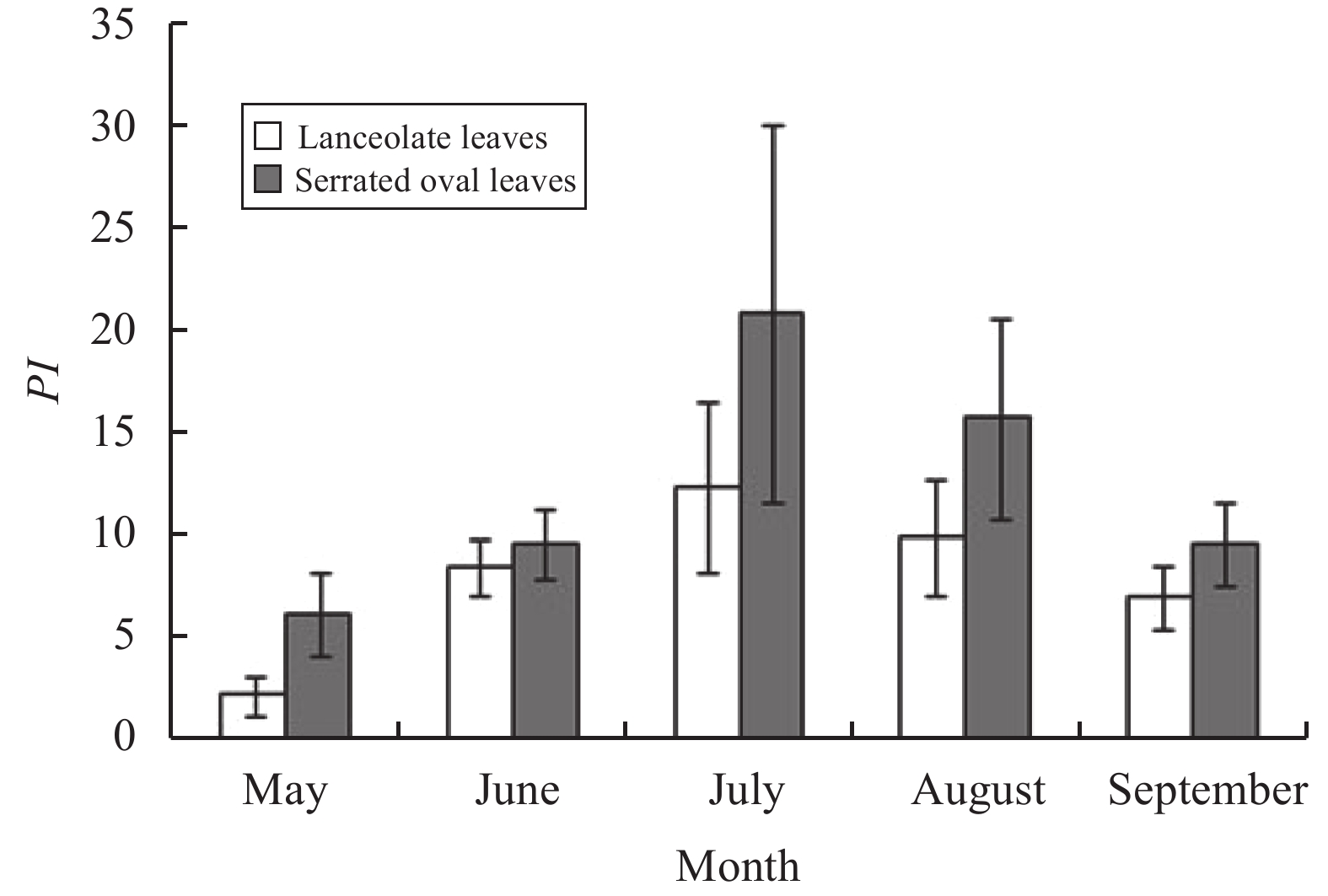
During the entire growing season (Figure 8), the light-energy-utilization parameters PI of serrate oval leaves are greater than those of lanceolate leaves. From May to September, the PI of lanceolate leaves is 33.7%, 87.0%, 59.1%, 62.9%, and 72.5% of the PI of serrate oval leaves, respectively. The seasonal change trend was consistent with the PI of the two kinds of leaves, shown as July > August > June > September > May.
|
Figure 8 Seasonal variation of light-energy-utilization parameters PI for heteromorphic leaves of Populus euphratica Oliver |
According correlation analysis results for the chlorophyll fluorescence kinetics parameters of P. euphratica (Table 1), there is no significant relationship between Fv/Fm, Fv/F0, ΦD0, PI, and RC/CS0; there was very significant correlation between Fv/Fm, Fv/F0, PI, and ΦD0. Fv/Fm, Fv/F0, and ΦD0 were negatively correlated with ET0/CS0, but not significantly. In addition, there were significant and very significant correlations between parameters.
|
|
Table 1 The correlation analysis of chlorophyll fluorescence-kinetics parameters in P. euphratica |
The chlorophyll fluorescence-induction kinetics curve rises from O to P, reflecting the photoreaction process. The changes from O to P are the further deoxidation and accumulation of PSII original electron-acceptor QA caused by the electrons released by water cleavage after illumination; the dark reaction cannot be started rapidly; the electron flow is slowed down through the PSII; thus the fluorescence is enhanced to P phase (Feng et al., 2002 ). Some experiments proved that QA and QB activity can affect the time in reaching the P phase and the fluorescence intensity of each phase (Schansker et al., 2011 ). Thus, there are some differences in the electron-transport activity between QA and QB in serrate oval and lanceolate leaves of P. euphratica. Vj and M0 reflect the accumulated amount of QA− and the relative speed of QA deoxidation, which together reflect the transmission of electrons between QA and QB (Strasserf and Srivastava, 1995; Srivastava et al., 1997 ). During the growing season, the Vj and M0 of serrate oval leaves were smaller than those of lanceolate leaves, indicating that serrate oval leaves have a higher electron transport rate between QA and QB, thus reducing the accumulation amount of QA− and increasing the photochemical reaction efficiency. Fv/Fm and Fv/F0 characterize the PSII initial light-energy-conversion efficiency and PSII potential activity, respectively (Zhang, 1999). Fv/Fm has a significant correlation with the quantum efficiency of the plant's CO2 assimilation (Ball et al., 1995 ). The PSII initial light-energy-conversion efficiency of serrate oval leaves is higher than that of lanceolate leaves; and the PSII potential activity is larger than that of lanceolate leaves: so serrate oval leaves have stronger carbon-assimilation ability in extremely arid environments of Ejina Oasis. This finding is consistent with those of Qiu et al. (2005) and Zheng et al. (2006) .
Based on the energy-flow model of Strasser and other energy-flow models, a part of the light energy (ABS) absorbed by the antenna pigment (Chl) is used for heat dissipation and fluorescence emission; and a part is captured by the reaction center (RC) to convert excitation energy into reducing energy; the QA is reduced to QA−, which is again oxidized to produce electron transport (Strasser et al., 2004 ). Various quantum efficiencies (such as ABS/RC, ET0/RC, DI0/CS0, and ET0/CS0) in a unit reaction center or light-receiving area, along with the number of reaction centers (RC/CS0) in a photosynthetic organ, can accurately reflect the photosynthetic organ's absorption, transformation, and dissipation of light energy (Li et al., 2005 ). Over the whole growing season, serrate oval leaves of P. euphratica have a greater reaction center density RC/CS0 than RC/CS0 and have higher ET0/CS0 but lower DI0/CS0 than lanceolate leaves. This pattern indicates that the specific activity of a unit cross-sectional area of serrate oval leaves is stronger than that of lanceolate leaves. Because lanceolate leaves have a small number of active reaction centers RC/CS0, the energy burden of a unit reaction center is heavier; lanceolate leaves take a strategy of increasing the quantum efficiency of unit reaction centers (ABS/RC, TR0/RC, ET0/RC, and DI0/RC) to adapt to the environment. In terms of energy distribution, the ΦP0, Ψ0, and ΦE0 of serrate oval leaves are greater than those of lanceolate leaves; and the ΦD0 of serrate oval leaves is smaller. This pattern indicates that serrate oval leaves have a higher proportion of open PSII reaction centers, which can capture or absorb more light energy for photochemical reaction and the electron-transport process; a small part of the energy is used for thermal dissipation, thereby producing more NADPH for carbon assimilation, proving that serrate oval leaves have a more efficient energy-distribution strategy. Light-energy-utilization parameter PI can demonstrate the ability of plants to utilize light energy and has been proven to be one of the most sensitive fluorescence parameters that can reflect a plant's photoreactivity (Stirbet, 2011). From May to September, the PI of P. euphratica serrate oval leaves is much higher than that of lanceolate leaves, indicating that serrate oval leaves have stronger photoreactivity.
During the growth season, seasonal changes in the chlorineophyll fluorescence kinetics of P. euphratica heteromorphic leaves can reflect the trend of functional changes of the photosynthetic structure of P. euphratica leaves from the early stages of development to the maturity and recession stages. According to seasonal changes and characteristics of Vj, M0, Fv/Fm, and Fv/F0 of P. euphratica serrate oval and lanceolate leaves, the two kinds of leaves have the minimum amount of QA− and the lowest relative speed of QA deoxidation in July, and have the highest primitive photochemical efficiency and PSII activity in July. The photosynthetic structures of P. euphratica heteromorphic leaves are in the development process in May and June, develop to the most perfect status in July, and gradually turn yellow due to changes in environmental conditions in September: their photosynthetic structures, especially the PSII function, are gradually inhibited, resulting in declining photochemical reaction efficiency in August and September. In July, P. euphratica heteromorphic leaves have the smallest number of reaction centers (RC/CS0) in a unit light-receiving area, because Ejina Oasis has the highest temperature and strongest light in July each year. P. euphratica leaves reduce the absorption of light energy by reducing the number of reaction centers, thus avoiding photoinhibition. Besides, the electron-transport energy of a unit reaction center (ET0/RC) shows an increasing trend, thus ensuring that P. euphratica still has high light-energy-conversion efficiency in July. The ΦP0, Ψ0, and ΦE0 of P. euphratica serrate oval and lanceolate leaves in July are larger than other months; but the ΦD0 of July is less than that of other months; this pattern indicates that P. euphratica leaves use more energy for photochemical reaction and electron-transfer process in July, thus producing more NADPH for carbon assimilation and proving that P. euphratica leaves have the optimal energy distribution in July. According to the general changes of chlorophyll fluorescence kinetics from May to September, the PSII reaction centers of P. euphratica have the strongest activity and the highest light-energy-conversion efficiency in July. P. euphratica have vigorous growth and the strongest resistance in July.
Fv/Fm, Fv/F0, and PI of P. euphratica have no significant correlation with PSII reaction center density RC/CS0 but have a very significant correlation with the energy-distribution parameters (ΦP0, Ψ0, ΦE0, and ΦD0). This pattern indicates that for P. euphratica leaves, light-energy-utilization efficiency is determined by the energy-distribution strategy of the leaves and is not directly related to the number of reaction centers in a unit cross-sectional area. However, the number of reaction centers can indirectly affect the light-energy utilization of P. euphratica by influencing the energy-distribution parameters.
Drought, high temperature, and other extreme natural conditions affect the plant's carbon-assimilation ability (Xu et al., 1992 ; Gong et al., 2005 ; Monneveux et al., 2006 ), resulting in a contradiction among photochemical reaction, electron transport, and heat dissipation (Qiu et al., 2011 ; Zhang et al., 2011 ; Chen et al., 2013 ). If the optimal energy distribution was achieved, it would take a dominant position under extreme environmental conditions. During the growth season, Fv/Fm, Fv/F0, Ψ0, ΦE0, and other parameters of P. euphratica serrate oval leaves were higher than those of lanceolate leaves, indicating that the light-energy-conversion efficiency of the antenna pigment and the activity of the PSII reaction centers in serrate oval leaves were significantly higher. There was more light energy for carbon assimilation, avoiding the accumulation of excess excitation energy, thereby enhancing the photosynthetic electron-transport capacity and increasing the ATP and NADPH required by photosynthetic carbon assimilation. Thus, enhancing the photosynthetic electron-transport capacity is an important reason why the photosynthetic rate of serrate oval leaves was higher than that of lanceolate leaves.
5 ConclusionsIn this paper, we compared the chlorophyll fluorescence-induction kinetics curves of two types of leaves (lanceolate and serrate oval leaves) of P. euphratica over the growth season. Results show that there were varied degrees of differences in the fluorescence signals of each phase between heteromorphic leaves in the same month, and the same kind of leaves in different months, as well as the length of time in reaching the P phase. During the growing season, Vj and M0 of serrate oval leaves were smaller than those of lanceolate leaves, indicating that serrate oval leaves have a higher electron transport rate between QA and QB, thus reducing the accumulation amount of QA− and increasing the photochemical reaction efficiency. Serrate oval leaves of P. euphratica have a greater reaction center density RC/CS0 than RC/CS0 and have higher ET0/CS0 but lower DI0/CS0 than lanceolate leaves. This pattern indicates that the specific activity of a unit cross-sectional area of serrate oval leaves is stronger than that of lanceolate leaves. Lanceolate leaves take a strategy of increasing the quantum efficiency of unit reaction centers (ABS/RC, TR0/RC, ET0/RC, and DI0/RC) to adapt to the environment. In terms of energy distribution, ΦP0, Ψ0, and ΦE0 of serrate oval leaves are greater than those of lanceolate leaves; and the ΦD0 of serrate oval leaves is smaller. Fv/Fm, Fv/F0, and PI of P. euphratica have no significant correlation with PSII reaction center density RC/CS0 but have a very significant correlation with the energy-distribution parameters (ΦP0, Ψ0, ΦE0, and ΦD0). Under extreme drought conditions, the energy-distribution and -utilization strategies of P. euphratica serrate oval leaves are more conducive to efficiently using the energy of P. euphratica and protecting its photosystem reaction center.
Acknowledgments:This work was supported by the Program for National Natural Science Foundation of China (31370396), and the Program for China Terrestrial Ecosystem Research Network (2017-LYPT-006).
Ball MC, Butterworth JA, Roden JS, et al. 1995. Applications of chlorophyll fluorescence to forest ecology. Australian Journal of Plant Physiology, 22(2): 311-319. DOI:10.1071/PP9950311 |
Chang ZQ, Feng Q, Su YH, et al. 2006. Photosynthetic characters of Populus euphratica leaves and response rate to light intensity and CO2 concentration in Ejina oasis of Northwest China
. Arid Land Geography, 29(4): 496-502. DOI:10.3321/j.issn:1000-6060.2006.04.007 |
Chen FL, Jin ZZ, Li SY, et al. 2013. Effects of heat stress on photosystem II in Karelinia Caspica
. Journal of Desert Research, 33(5): 1371-1376. DOI:10.7522/j.issn.1000-694X.2013.00201 |
Chen HX, Gao HY, An SZ, et al. 2004. Dissipation of excess energy in Mehler-peroxidase reaction in Rumex leaves during salt shock. Photosynthetica, 42(1): 117-122. DOI:10.1023/B:PHOT.0000040579.37842.ca |
Dai J, Gao H, Dai Y, et al. 2004. Changes in activity of energy dissipating mechanisms in wheat flag leaves during senescence. Plant Biology, 6(2): 171-177. DOI:10.1055/s-2004-817845 |
Feng JC, Hu XL, Mao XJ. 2002. Application of Chlorophyll fluorescence dynamics to plant physiology in adverse circumstance. Economic Forest Researches, 20(4): 14-18. DOI:10.3969/j.issn.1003-8981.2002.04.004 |
Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA)-General Subjects, 990(1): 87-92. DOI:10.1016/S0304-4165(89)80016-9 |
Gong JR, Zhao AF, Su PX, et al. 2005. Comparative study on photosynthetic characteristics of several dominant plants in Heihe Drainage Basin. Journal of Desert Research, 25(4): 587-592. DOI:10.3321/j.issn:1000-694X.2005.04.023 |
Li PM, Gao HY, Strasser RJ. 2005. Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study. Journal of Plant Physiology and Molecular Biology, 31(6): 559-566. DOI:10.3321/j.issn:1671-3877.2005.06.001 |
Monneveux P, Rekika D, Acevedo E, et al. 2006. Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes. Plant Science, 170(4): 867-872. DOI:10.1016/j.plantsci.2005.12.008 |
Nie L, Li SY, Liao XR, et al. 1999. Chlorophyll fluorescence characteristics and its relationship with mineral element contents in leaves of Shatianyou pomelo variety. Journal of Fruit Science, 16(4): 284-288. DOI:10.3969/j.issn.1009-9980.1999.04.011 |
Peng JG, Jiang XR, Xu J, et al. 2017. Underestimated chlorophyll a fluorescence measurements on Buxus microphylla red winter leaves
. Photosynthetica, 55(4): 561-567. DOI:10.1007/s11099-016-0660-5 |
Qiu CH, Ji WW, Guo YP. 2011. Effects of high temperature and strong light on chlorophyll fluorescence, the D1 protein, and Deg1 protease in Satsuma mandarin, and the protective role of salicylic acid. Acta Ecologica Sinica, 31(13): 3802-3810. |
Qiu J, Zheng CX, Yu WP. 2005. Comparison of photosynthetic rate and fluorescence characteristics of heteromorphism leaf of Populus euphratica
. Jilin Forestry Science and Technology, 34(3): 19-21. DOI:10.3969/j.issn.1005-7129.2005.03.007 |
Said SA, Torre F, Derridj A, et al. 2013. Gender, mediterranean drought, and seasonality: Photosystem II photochemistry in Pistacia lentiscus L
. Photosynthetica, 51(4): 552-564. DOI:10.1007/s11099-013-0055-9 |
Schansker G, Tóth SZ, Kovács L, et al. 2011. Evidence for a fluorescence yield change driven by a light-induced conformational change within photosystem II during the fast chlorophyll a fluorescence rise. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1807(9): 1032-1043. DOI:10.1016/j.bbabio.2011.05.022 |
Schreiber U, Bilger W, Neubauer C, 1994. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM (eds.). Ecophysiology of Photosynthesis. Berlin, Heidelberg: Springer, pp. 49–70. DOI: 10.1007/978-3-642-79354-7_3.
|
Srivastava A, Guissé B, Greppin H, et al. 1997. Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP
. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1320(1): 95-106. DOI:10.1016/S0005-2728(97)00017-0 |
Stirbet A. 2011. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient
. Journal of Photochemistry and Photobiology B: Biology, 104(1–2): 236-257. DOI:10.1016/j.jphotobiol.2010.12.010 |
Strasser RJ, Tsimilli-Michael M, Srivastava A, 2004. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds.). Chlorophyll A Fluorescence. Dordrecht: Springer, pp. 321–362. DOI: 10.1007/978-1-4020-3218-9_12.
|
Strasserf RJ, Srivastava A. 1995. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochemistry and Photobiology, 61(1): 32-42. DOI:10.1111/j.1751-1097.1995.tb09240.x |
Su PX, Zhang LX, Du MW, et al. 2003. Photosynthetic character and water use efficiency of different leaf shapes of Populus euphratica and their response to CO2 enrichment
. Acta Phytoecologica Sinica, 27(1): 34-40. DOI:10.17521/cjpe.2003.0005 |
Su YH, Feng Q, Lü SH, et al. 2004. The degradation of ecological environment in Ejinaqi and its cause analysis. Plateau Meteorology, 23(2): 264-270. DOI:10.3321/j.issn:1000-0534.2004.02.021 |
Wang HL, Yang SD, Zhang CL. 1998. The photosynthetic characteristics of differently shaped leaves in Populus euphratica Olivier
. Photosynthetica, 34(4): 545-553. DOI:10.1023/A:1006813513228 |
Wang HZ, Han L, Xu YL, et al. 2011. Response of chlorophyll fluorescence characteristics of Populus euphratica heteromorphic Leaves to high temperature
. Acta Ecologica Sinica, 31(9): 2444-2453. |
Wang SJ, Chen BH, Li HQ, 1995. The Populus euphratica Forests. Beijing: China Environmental Science Press, pp. 141–144.
|
Xia JB, Zhang GC, Wang RR, et al. 2014. Effect of soil water availability on photosynthesis in Ziziphus jujuba var
. spinosus in a sand habitat formed from seashells: comparison of four models. Photosynthetica, 52(2): 253-261. DOI:10.1007/s11099-014-0030-0 |
Xu DQ, Zhang YZ, Zhang RX. 1992. Photoinhibition of photosynthesis in Plants. Plant Physiology Communications, 28(4): 237-243. DOI:10.13592/j.cnki.ppj.1992.04.001 |
Zhang SR. 1999. A discussion on chlorophyll fluorescence kinetics parameters and their significance. Chinese Bulletin of Botany, 16(4): 444-448. DOI:10.3969/j.issn.1674-3466.1999.04.021 |
Zhang YJ, Xie ZK, Zhao XY, et al. 2011. Effects of water stress on photosynthetic characteristics, Chlorophyll fluorescence, and dry matter of Oriental Lilies. Journal of Desert Research, 31(4): 884-888. |
Zhao CM, Wang GX. 2002. Effects of drought stress on the photoprotection in Ammopiptanthus mongolicus Leaves
. Acta Botanica Sinica, 44(11): 1309-1313. DOI:10.3321/j.issn:1672-9072.2002.11.008 |
Zheng CX, Qiu J, Jiang CN, et al. 2006. Comparison of characteristics of stomas and photosynthesis of Populus euphratica polymorphic leaves
. Scientia Silvae Sinicae, 42(8): 19-24. DOI:10.3321/j.issn:1001-7488.2006.08.004 |
 2018, Vol. 10
2018, Vol. 10
