2. University of Chinese Academy of Sciences, Beijing 100049, China
The soil-related obstacles to continuous cropping of economic crops have a negative impact on the yield and quality of crops. Lanzhou lily is a variant of Lilium davidii Duchartre, a perennial herbaceous bulb plant. It is an ornamental plant that can also be cultivated for medicinal purposes. It is not only a high-grade Chinese lily but also is the only sweet lily (Ma et al., 2005 ) and very valuable for planting. It is suitable to be grown in areas with large day-to-night temperature differences. However, during the planting process of Lanzhou lily, there are some serious obstacles to continuous cropping that negatively affect the yield and quality.
Most of the crop loss in agricultural production can be attributed to two main causes: (1) the change of soil properties and rhizosphere microbiome and (2) the plant allelopathy effects (Chen et al., 2011 ). Studies have found that root exudates played an important role in disorders found with continuous cropping, and some components of the root exudates exerted a toxic effect on the plant itself. Besides, by changing the structure and composition of the rhizosphere microbes, root exudates could also affect plant growth negatively (Kourtev et al., 2003 ). Similarly, the growth of taros was hindered by root exudates, thus seriously affecting its yield (Asao et al., 2003 ). Studies have shown that many phenolic acids are inhibitors of crop growth (Bertin et al., 2003 ; Seastedt et al., 2008 ; Iannucci et al., 2013 ). Wu et al. found that the toxicity of phthalic acid and adipic acid was the strongest in the root exudates of lily, and the concentration of phthalic acid in the soil increased with the extension of the tillage period. In the tissue culture of lily, phthalic acid decreased the root of Lanzhou lily, as well as the fresh weight of both plant and seed, leading to oxidative stress at the root of the plant. In the process of culturing fungi, it was found that phthalic acid promoted the production of mycotoxins at low concentrations (Wu et al., 2015 ).
Root exudates are a key factor in regulating the vitality of rhizosphere microflora (Wu et al., 2014 ). Soil microbes occupy an important position in the ecosystem and participate in the transformation of soil nutrients and organic matter (Benayas et al., 2009 ), regulating the growth of plants. Recent studies have found that imbalances of soil microflora and diversity are the underlying causes of soil-borne diseases (Ren et al., 2008 ). Lily fusarium wilt caused by soil-borne pathogens is a major obstacle to lily cultivation, which also is one of the most important factors in obstacles to the continuous cropping of Lanzhou lily.
Currently, intercropping is a relatively effective way to deal with obstacles to continuous cropping. Reasonable intercropping not only can take full advantage of sunlight and moisture but also improve the crop rhizosphere environment. Maize is a very common crop in Northwest China and is often interplanted with other crops (Su et al., 2013 ). Meanwhile, root exudates can provide nutrients for plant growth and improve the plant's absorptive capacity of nutrition, while also having interspecies identification, signal transmission, and other functions (Haichar et al., 2014 ). Based on the characteristics of root exudates, we studied the changes of structure and composition of rhizosphere microbial communities and root exudates in lily–maize intercropping through the function of the signal-transduction capacity of root exudates.
2 Materials and methodsThe root soil used for the research was selected from the experimental plots of Longde County of the Ningxia Hui Autonomous Region (35°38′N, 106°06'E). There were three treaments with 3.8m×4.8m per plot, three replications, and a randomized complete block design. The arrangement of plots was as follows: The planting pattern of the sample was maize:lily = 2:4, namely two rows of maize intercropping four lilies each line. The spacing between the two rows of maize was 40 cm; the spacing between the lilies was 80 cm. Lily placement used the pattern of furrows and maize planting with plastic film mulch. In the monoplanting system, maize was cultured using a wide–narrow row pattern-narrow-row planting of two rows of maize, with the spacing between the two rows being 40 cm, and the spacing between two successive maize plants being 27 cm. In culturing lily using the pattern of furrows, the spacing between the two furrows was 40 cm; and the spacing between two successive lilies was 22 cm. We collected the rhizosphere soils of maize monoplanting, lily monoplanting, and lily–maize intercropping for root exudates and soil microbial analysis.
2.1 Rhizosphere microbial diversity analysisThe rhizosphere soils of single lily (SL), single maize (SM), intercropped maize (IM), and intercropped lily (IL) were used for the analysis of microbial diversity, with each sampling having three replicates. First, we extracted genome DNA. Total genome DNA from samples was extracted using the CTAB/SDS method. Second, we monitored the DNA concentration and purity on 1% agarose gels. According to the concentration, we used sterile water to dilute the DNA to 1 ng/μL.
2.1.1 Amplicon generationPrimer of 16S used were V4: 515F-806R, the primer of 18S used were V4: 528F-706R and V9: 1380F-1510R, ITS1's primer used were ITS1F-ITS2. The 16S/18S rRNA genes were amplified using the specific primer with the barcode. All PCR (Polymerase Chain Reaction) reactions were carried out in 30-μL reactions with 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μmol/L of forward and reverse primers, and about 10 ng template DNA. Thermal cycling consisted of initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s. Finally, temperature was held at 72 °C for 5 min.
2.1.2 PCR products quantification and qualificationWe mixed the same volume of 1X loading buffer (containing SYB green) with PCR products and performed electrophoresis on 2% agarose gel for detection. Samples with a bright main strip between 400 and 450 bp were chosen for further experiments.
2.1.3 PCR products mixing and purificationPCR products were mixed in equidensity ratios. Then, the mixture of PCR products was purified with a GeneJET Gel Extraction Kit (Thermo Scientific).
2.1.4 Library preparation and sequencingSequencing libraries were generated using NEB Next® UltraTM DNA Library Prep Kit for Illumina (NEB, USA), following the manufacturer's recommendations; and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. At the last, the library was sequenced on an Illumina MiSeq platform; and 250 bp/300 bp paired-end reads were generated.
2.2 Root exudates collection, purification, concentration, and analysisWe collected three rhizosphere soils of the plants under different culturing patterns–single lily (SL), single maize (SM), and maize intercropping with lily (L×M)—and stored them in a low-temperature refrigerator at −80 °C. After treatment, the type and composition of root exudates were analyzed by GC/ TOFMS. Samples were taken into the 2-mL EP tubes. The contents of the samples shown in Table 1 were extracted with 0.4 mL extraction liquid (Vmethanol:
|
|
Table 1 Number and weight of sample |
GC/TOFMS analysis was performed using an Agilent 7890 gas chromatograph system coupled with a Pegasus HT time-of-flight mass spectrometer. The system utilized a DB-5MS capillary column coated with 5% diphenyl cross-linked with 95% dimethylpolysiloxane (30m×250μm inner diameter, 0.25-μm film thickness; J&W Scientific, Folsom, CA, USA). A 1-μL aliquot of the analyte was injected in splitless mode. Helium was used as the carrier gas, the front inlet purge flow was 3 mL per minute, and the gas flow rate through the column was 1 mL per minute. The initial temperature was kept at 50 °C for 1 min, raised to 330 °C at a rate of 10 °C per minute, then kept for 5 min at 330 °C. The injection, transfer-line, and ion-source temperatures were 280, 280, and 250 °C, respectively. The energy was −70 eV in electron impact mode. The mass spectrometry data were acquired in full-scan mode with the m/z range of 30–600 at a rate of 20 spectra per second after a solvent delay of 6.1 min.
The stability of the system was measured by the retention time of the internal standard (ribitol). Accordingly, the internal standard retention time of three samples had a standard deviation of 0.001721, indicating that the machine detection system was very stable. Chroma TOF 4.3X software of the LECO Corporation and the LECO-Fiehn Rtx5 database were used for raw peaks exacting, the data-baselines filtering and calibration of the baseline, peak alignment, deconvolution analysis, peak identification, and integration of the peak area (Kind et al., 2009 ).
2.3 Data analysisData were analyzed statistically using EXCEL, Origin, R language, and SPSS.
3 Results 3.1 Summary of ITS1-sequencing classification under different planting patternsSamples were sequenced using the Illumina HiSeq sequencing platform. The labels and primer information of the ITS1 sequencing are shown in Table 2. There were 702,004 reads from 12 samples after sequencing and 648,087 tags after splicing. The average length of the tags was 241 bp. After filtering the chimeric, the number of effective tags was 50,771 and the GC% average base was 54.95%. After removing redundancy, removing the single unique tags sequence, clustering the effective tags from all samples, and clustering the sequences into OTUs with 97% identity, the number of samples OTU was between 397 and 650. A representative sequence of the OTUs is annotated, and there are 603,962 taxon tags got the comment information.
|
|
Table 2 Label and primer information of ITS1 sequencing |
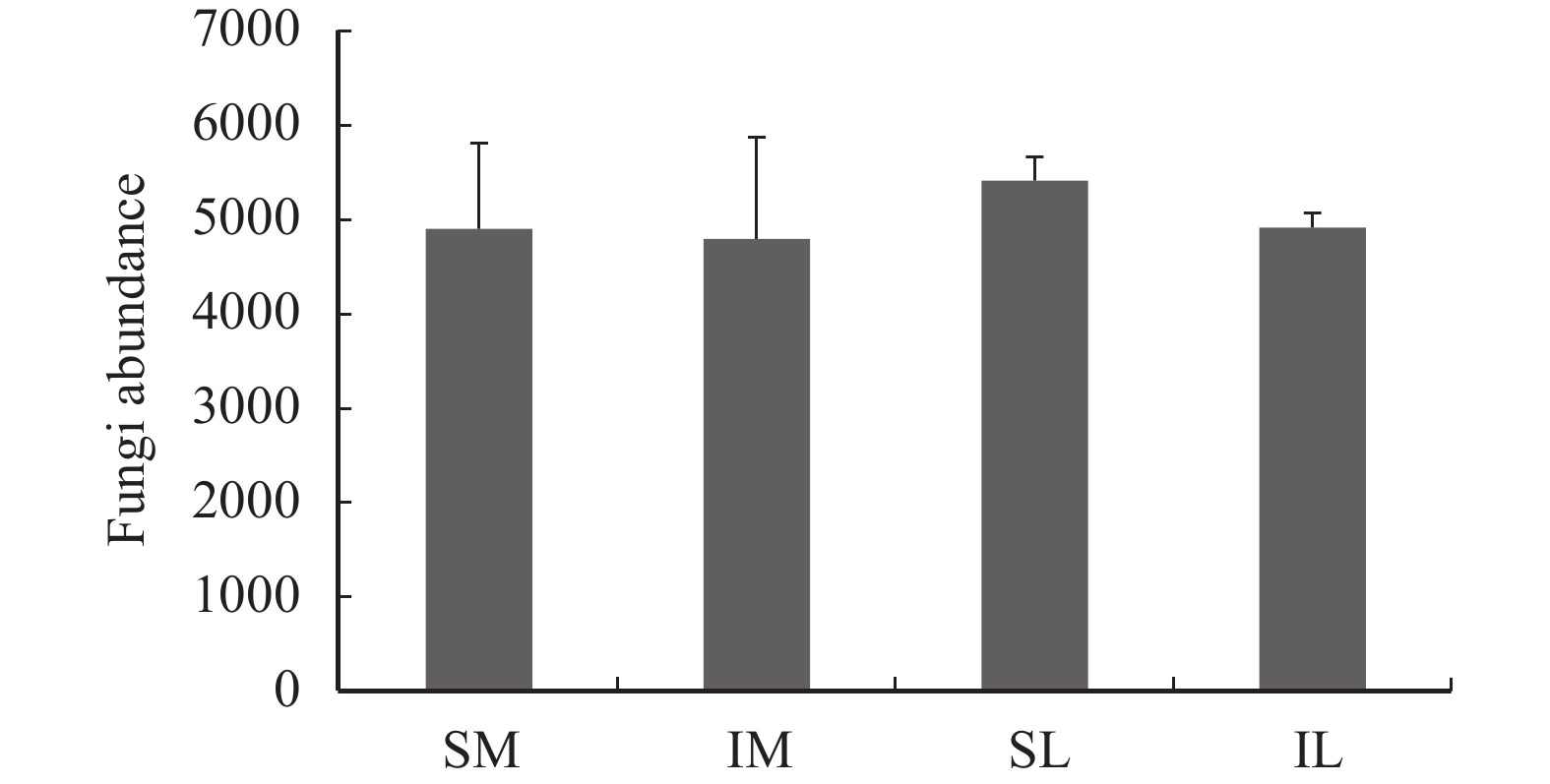
For the rarefaction curve (Figure 1) obtained after ITS1 sequencing, the trends indicate that the amount of sequencing data is reasonable. The total abundance of fungi in SL, IL, SM, and IM by species annotation is shown in Figure 2. After interplanting, the total abundance of fungi in IL and IM was significantly lower than that in SL and SM, respectively.

|
Figure 1 Rarefaction curve for fungi in all samples (SM: the root soil of the single-maize system; SL: the root soil of the single-lily system; IM: the root soil from maize of the intercropping system; IL: the root soil from lily of the intercropping system. 1, 2, 3 denote three repetitions) |
|
Figure 2 The fungal abundance of all samples (SM: the root soil of the single-maize system; SL: the root soil of the single-lily system; IM: the root soil from maize of the intercropping system; IL: the root soil from lily of the intercropping system) |
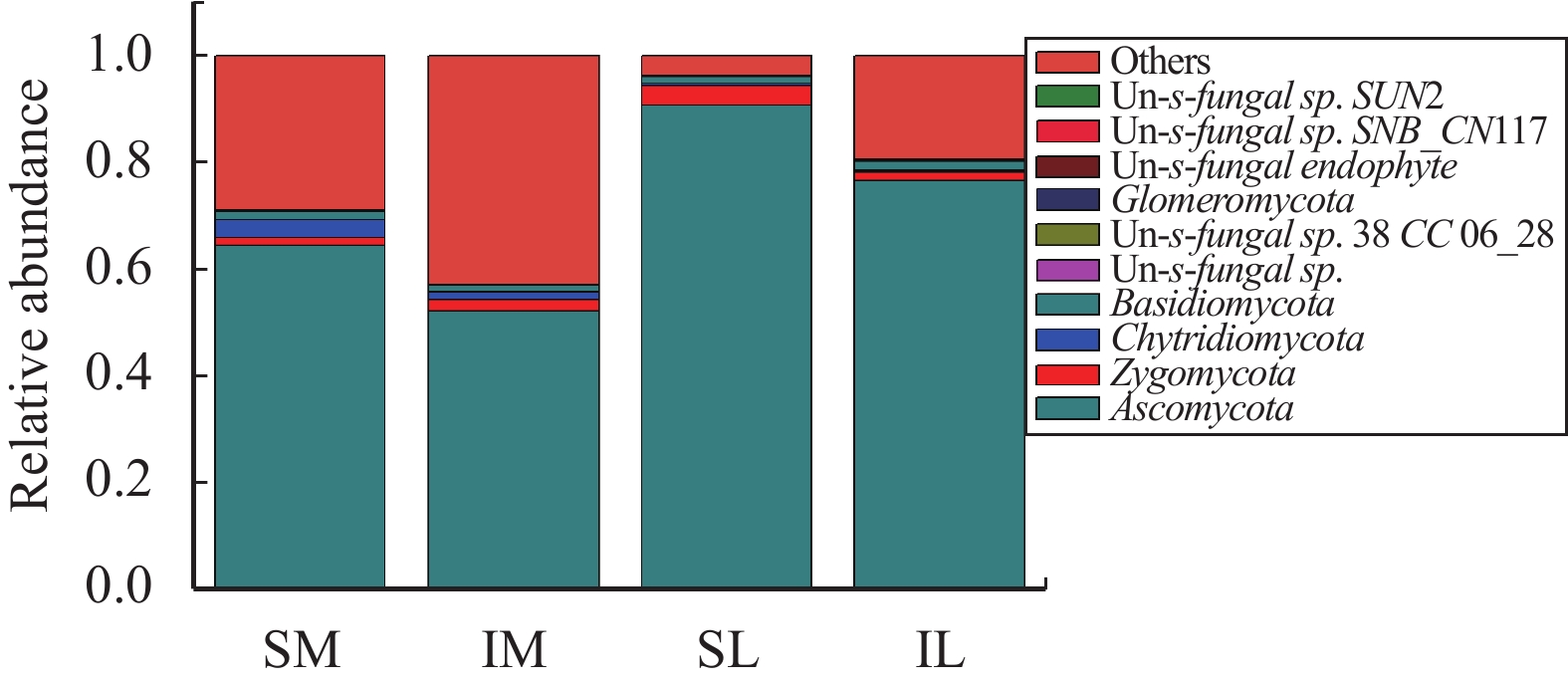
The composition of fungal communities in all samples was similar, except for the proportions of the components. After OTU clustering, the relative abundance of the components of the fungus at the phylum classification level (Figure 3) showed that the relative abundance of Ascomycota was the highest among the top-ten relative abundances. With monoculturing, the relative abundance of Ascomycota in the rhizosphere soil of SL1 was 96.30%; and the relative abundance in maize rhizosphere soil was 95.54%. After intercropping, the relative abundance of Ascomycota in the rhizosphere soil of IL1 and IM1 was the highest, 76.57% and 93.81%, respectively. The relative abundance of Ascomycota includes the majority of fungi in rhizosphere soil, followed by the relative abundance of Chytridiomycota and Zygomycota; but the total relative abundance of the other two is less than 10%.
|
Figure 3 Relative abundance of each sample fungi at the phylum level (SM: the root soil of the single-maize system; SL: the root soil of the single-lily system; IM: the root soil from maize of the intercropping system; IL: the root soil from lily of the intercropping system) |
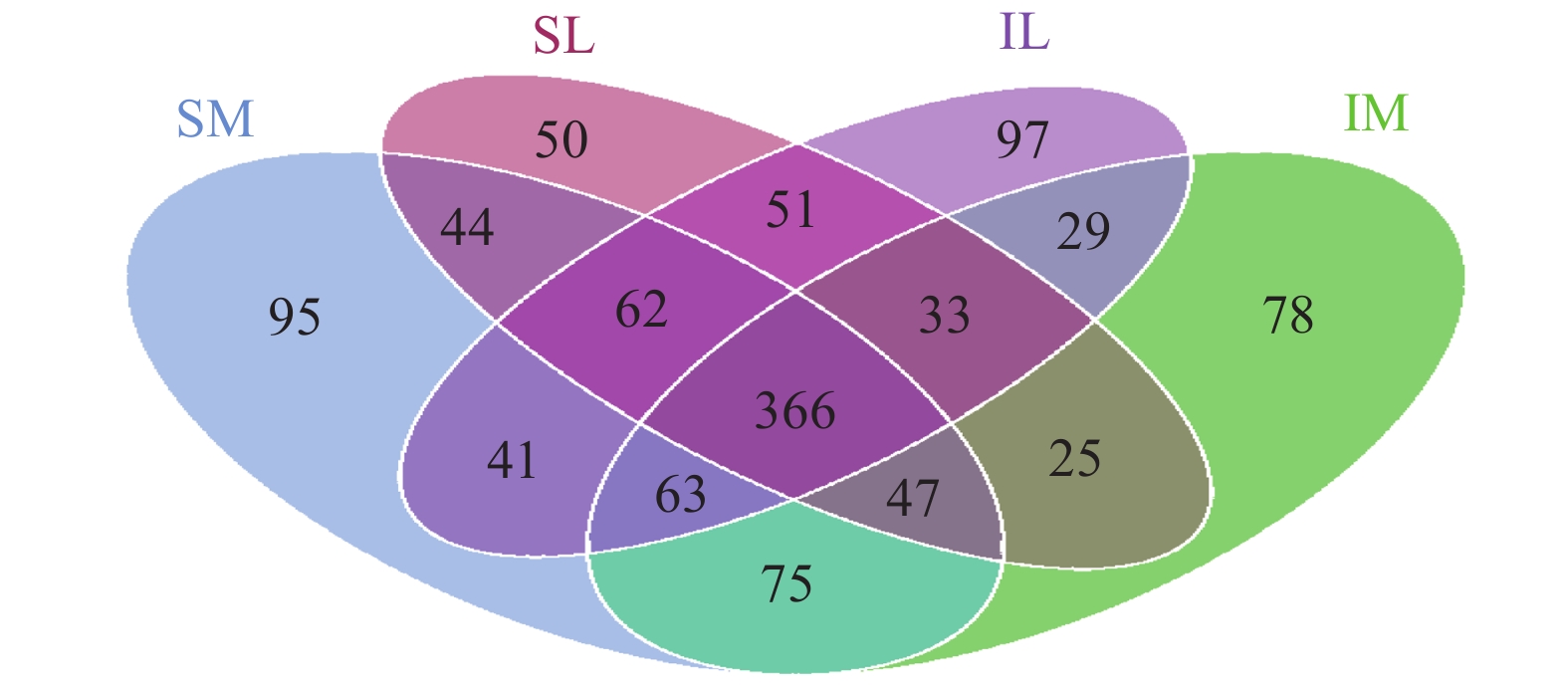
The total number of OTUs for all fungi in the four samples was 2,929 (Figure 4), whereas with intercropping, the unique OTU is 29; with monoculture, unique OTU was 44; and the OTU in the 12 samples was 366. In the unique OTU of the interplanting system, Pleosporales sp., Scleroderma sp., and Chaetomium sp., which had relatively high abundance in the rhizosphere soil of lily when interplanted, accounted for 23.21% of the OTU. In the unique OTU of the monoculturing system, Verticillium sp. and Zygopleurage sp., which had relatively high abundance in the rhizosphere soil of lily when monocultured, accounted for 19.79% of the OTU.
|
Figure 4 Four-way Venn diagram showing the unique fungal OUTs of samples (SM: the root soil of the single-maize system; SL: the root soil of the single-lily system; IM: the root soil from maize of the intercropping system; IL: the root soil from lily of the intercropping system) |
The differences in fungal diversity about SM–IM and SL–IL were analyzed by the Wilcoxon test, with the results shown in Table 3. There was no significant difference in the index between SL and IL. However, the Shannon index and the Simpson index for the IL were higher than for the SL.
|
|
Table 3 The fungi richness index and diversity index of SM–IM and SL–IL rhizosphere soil (Mean±SD) |
Sequencing was performed using the Illumina HiSeq sequencing platform, with the labels and primer information of 16S rDNA sequencing as shown in Table 4. We obtained 621,236 reads from 12 samples, 612,585 tags after splicing, and an average tag length of 253 bp. After chimerization and filtration, the number of effective tags was 48,984; and the average content of GC% was 55.80%. Redundancy and the single unique tags were removed. Then, the effective tags of all the samples were clustered into OTUs by 97.9% identities; and the number of the samples' OTUs is between 2,770 and 3,408. A representative sequence of the OTUs was annotated, and the taxon tags for the comment information were 544,888.
|
|
Table 4 Label and primer information of 16S rDNA sequencing |
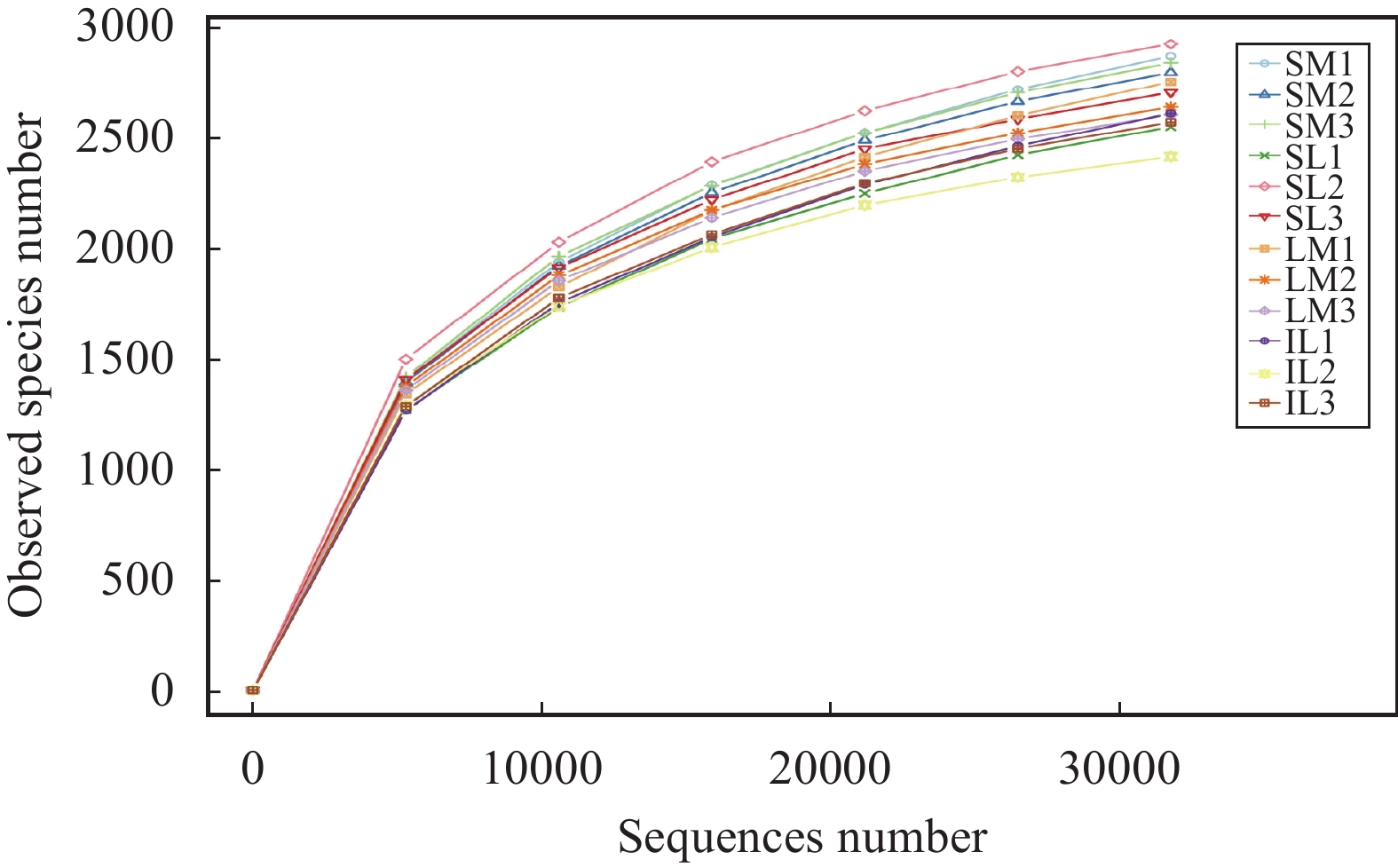
The rarefaction curve (Figure 5) obtained after 16S rDNA sequencing rose from 0 to 2,500, and the curve was finally flattened to indicate that the sequencing data was reasonable.
|
Figure 5 Rarefaction curve for bacteria in all samples (SM: the root soil of the single-maize system; SL: the root soil of the single-lily system; IM: the root soil from maize of the intercropping system; IL: the root soil from lily of the intercropping system. 1, 2, 3 denote three repetitions) |
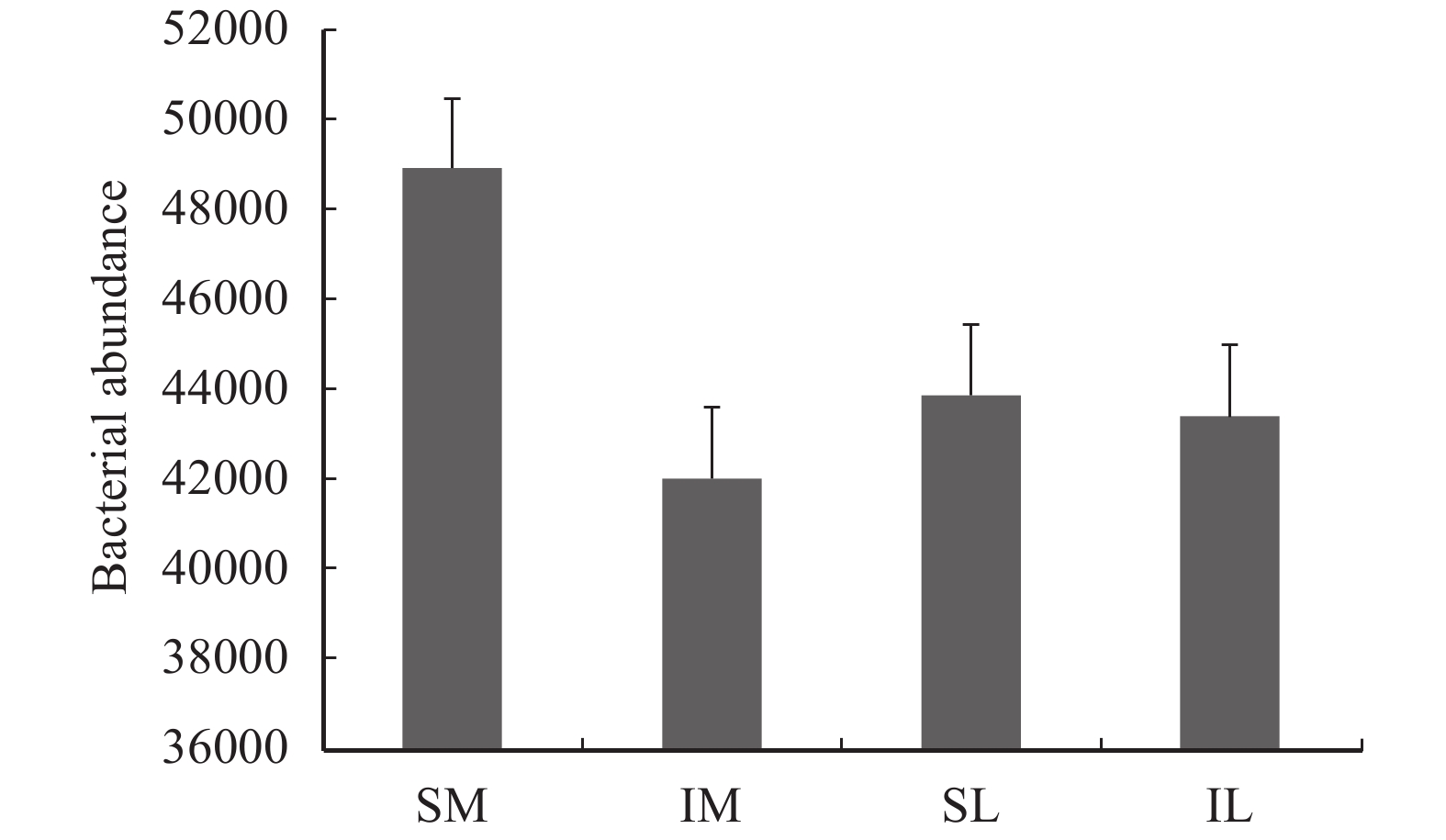
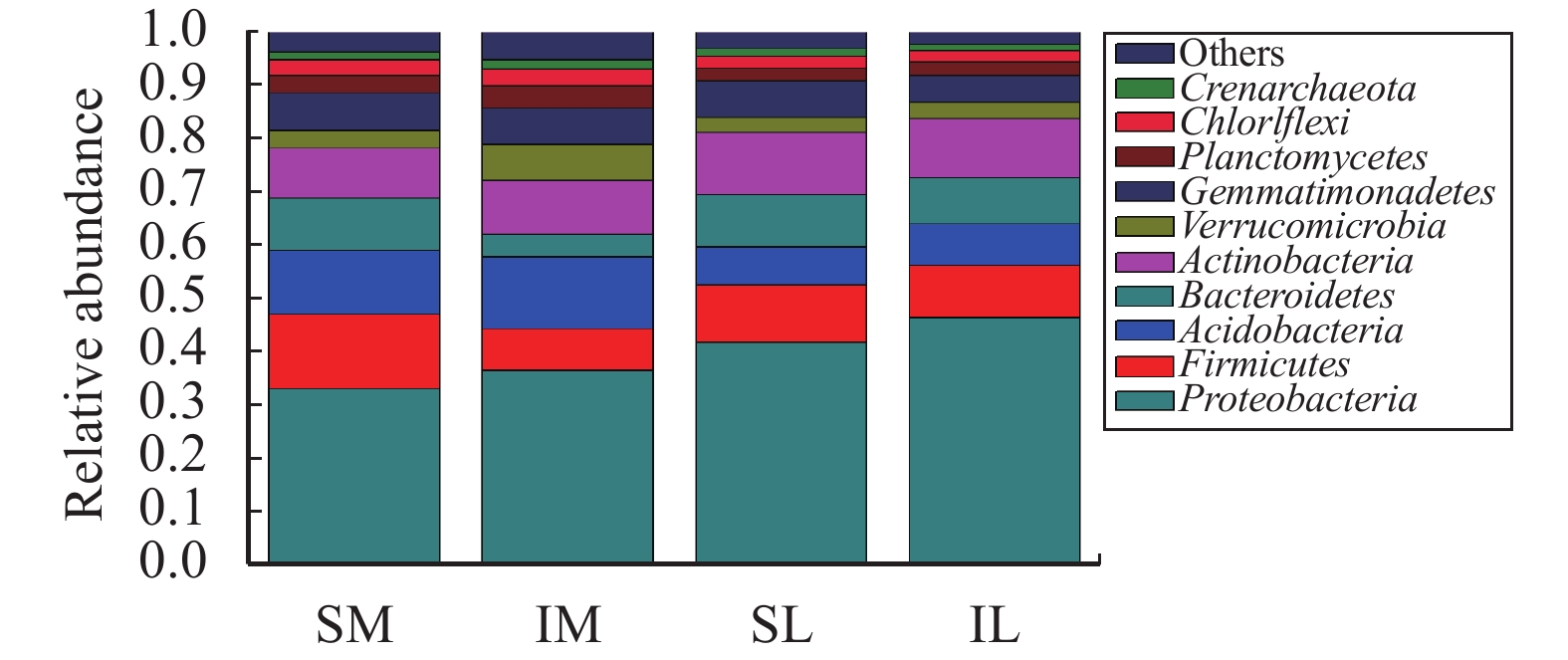
The total abundance of lily and maize rhizosphere bacteria was obtained by annotation (Figure 6), and the total amount of lily and maize rhizosphere bacteria with interplanting decreased in comparison to monoculturing. Through the OTU clustering, the species and relative abundance of the top-ten bacteria in the lily and maize roots at the phylum classification level under different planting patterns are shown in Figure 7. Proteobacteria is dominant; in the monoculturing system, the relative abundance of Proteobacteria in maize and lily roots was 33.17% and 41.82%, respectively. With interplanting, the relative abundance of Proteobacteria in lily and maize rhizospheres increased to 36.55% and 46.42%, respectively. In addition, with interplanting, the relative abundance of Acidobacteria, Verrucomicrobia, and Planctomycetes in the roots of the two plants was increased. The relative abundance of Firmicutes, Bacteroidetes, and Gemmatimonadetes decreased in the two-crop rhizosphere, as compared to monoculturing. The relative abundance of Actinobacteria, Chloroflexi, and Crenarchaeota in maize roots was higher than that with monocropping; and the relative abundance of these bacteria in lily decreased during intercropping.
|
Figure 6 The bacteria abundance of all samples (SM: the root soil of the single-maize system; SL: the root soil of the single-lily system; IM: the root soil from maize of the intercropping system; IL: the root soil from lily of the intercropping system) |
|
Figure 7 Relative abundance of each sample bacteria at the phylum level (SM: the root soil of the single-maize system; SL: the root soil of the single-lily system; IM: the root soil from maize of the intercropping system; IL: the root soil from lily of the intercropping system) |
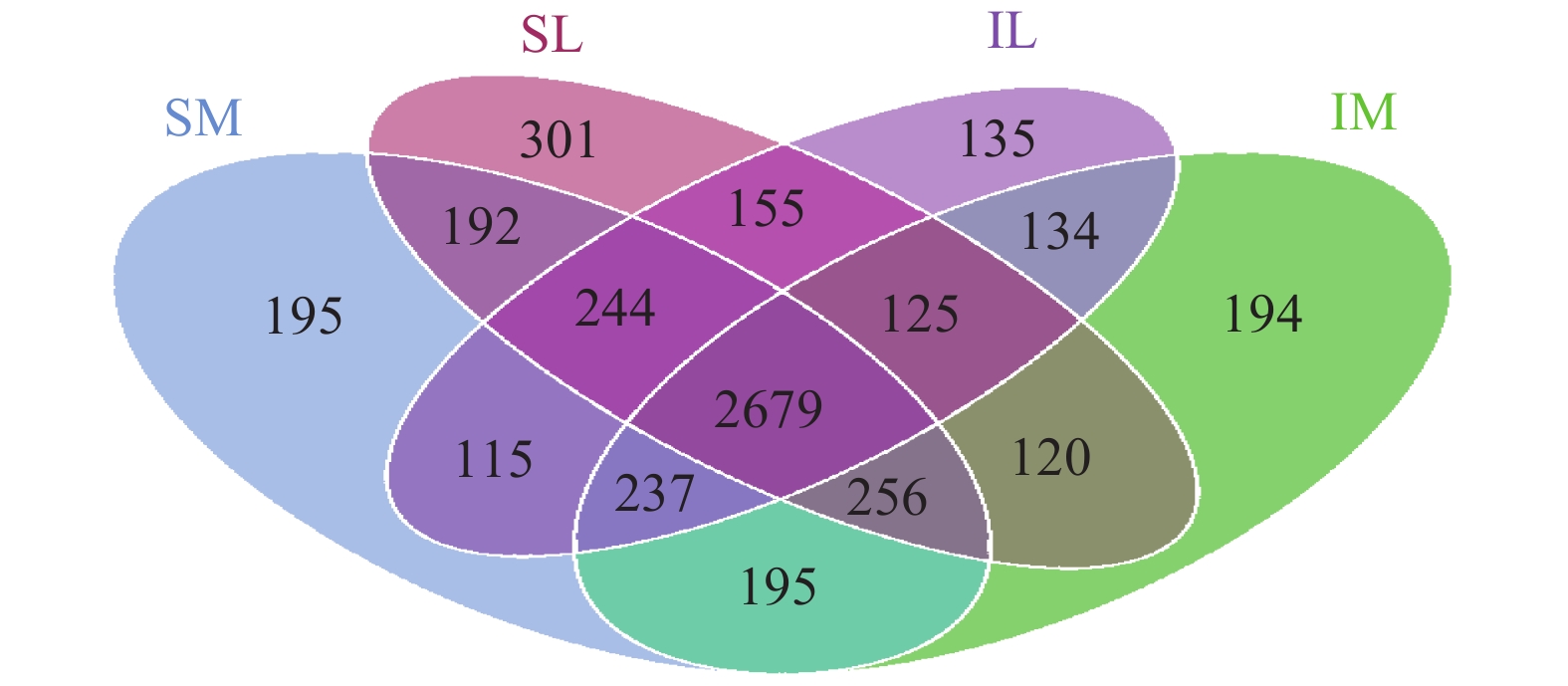
The total number of OTUs for all bacteria in the four samples was 15,949 (Figure 8). The unique OTU is 134 with intercropping, monoculture unique OTU is 192 and the OTU in the 12 samples was 2,679. In the unique OTU of the interplanting system, Clostridium sp., Fimbriimonas sp., Coprococcus sp., and Flavobacterium sp., which had relatively high abundance in the rhizosphere soil of lily when interplanted, accounted for 11.24% of the OTU. In the single OTU, Allobaculum sp. and Planctomyces sp., which had relatively high abundance in the rhizosphere soil of lily with monoculturing, accounted for 11.88% of the total OTU.
|
Figure 8 Four-way Venn diagram showing the unique bacteria OUTs of samples (SM: the root soil of the single-maize system; SL: the root soil of the single-lily system; IM: the root soil from maize of the intercropping system; IL: the root soil from lily of the intercropping system) |
The differences of bacterial diversity about SM–IM and SL–IL were analyzed using the Wilcoxon test; the results are shown in Table 5. By analyzing the diversity of lily rhizosphere bacteria under monoculturing and interplanting, it was found that the Shannon and Simpson indexes were significantly different. The results showed significant differences in microbial diversity among the roots of lily under different planting patterns. The abundance and diversity of the soil microbes of the lily rhizospheres with interplanting were lower than those with monoculturing.
|
|
Table 5 The bacterial Richness index and Diversity index of SM-IM and SL-IL rhizosphere soil (Mean±SD) |
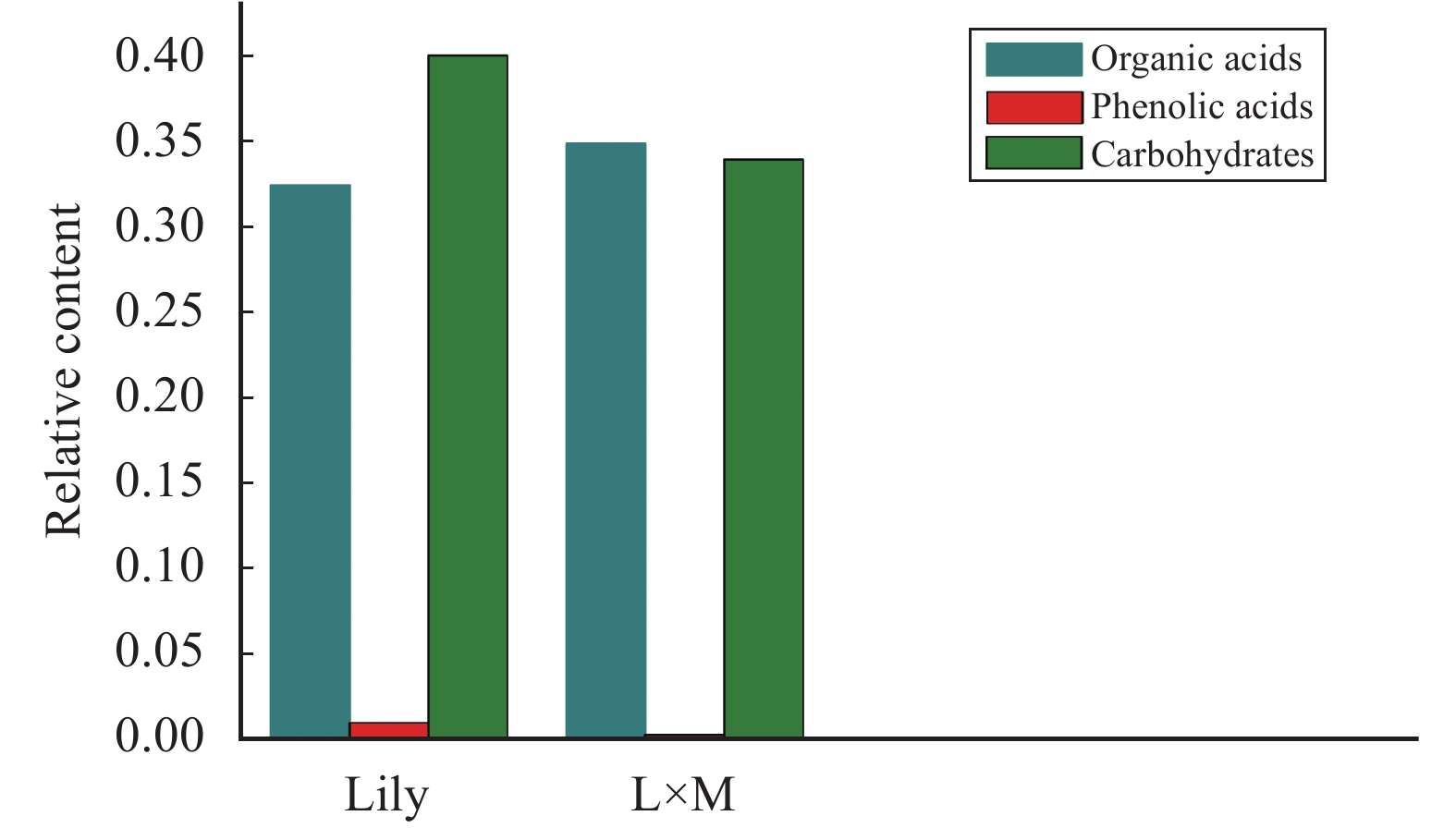
Using GC–MS analysis of the root exudates from single-maize (Maize), single-lily (Lily), and lily–maize interplanting (L×M), we found that the species of root exudates around the three samples were 193, 208, and 163, respectively; and the number of organic acids was 67, 70, and 53, respectively. Seven kinds of phenolic acids were detected in Maize, nine kinds in lily, and six kinds in L×M. The number of carbohydrate substances was 26, 27, and 21, respectively. The relative contents of organic acids, phenolic acids, and carbohydrates in the three rhizosphere soils are shown in Figure 9. The amount of substance contained in the root exudates after interplanting was reduced, as compared with that of monoculturing; and the types of organic acids, phenolic acids, and carbohydrates were also reduced. The content of organic acids in the root exudates of lily was lower than that of L×M root exudates. After interplanting, the relative content of phenolic acid decreased; and the cis-sinapinic acid and cumic acid detected in the root exudates of lily was not found in the root exudates of L×M. The relative content of carbohydrates after interplanting decreased, as compared with that of lily.
|
Figure 9 The relative contents of organic acids, phenolic acids, and carbohydrates in samples (Lily: the root exudates of the single-lily system; L×M: the root exudates of the intercropping system) |
Different planting patterns can alter the community structure and diversity of soil microbes (Sanguin et al., 2009 ). High vegetation diversity can increase the carbon source in the soil, which can provide a more complex growing space for microbes (Thevathasan and Gordon, 2004). Therefore, we hope that the community structure of lily root microbes can be changed by changing the planting pattern of lily, which can alleviate the effect of continuous cropping. In the present study, the rarefaction curve about ITS1 fungi sequencing and 16S rDNA bacterial sequencing tends to balance; therefore, the data sizes of the two groups are reasonable. By analyzing the abundance of fungi and bacteria at the kingdom level, it was found that the composition of bacteria and fungi in the soil of lily and maize roots at the phylum level did not change (Figure 3 and Figure 6). But the abundance of bacteria and fungi decreased after interplanting (Figure 2 and Figure 5). The fungi associated with continuous cropping are Pythium sp. (Sewell, 1981; Jaffee et al., 1982 ), Cylindrocarpon sp. (Jaffee et al., 1982 ; Braun, 1991, 1995), Phytophthora sp. (Sutton et al., 1981 ), Armillaria mellea (Sutton et al., 1981 ), Peniophora sacrata (Taylor and Wallace, 1970), Trichoderma hamatum, Torulomyces lagena, Motierella sp. (Utkhede et al., 1992 ), and Funneliformis sp. (Hou, 2012). In addition, Fusarium sp. commonly cause lily and other herbaceous plants to suffer from fusarium wilt (Gullino et al., 2015 ). In this study, we measured the fungi Cylindrocarpon sp., Trichoderma sp., Fusarium sp., and Funneliformis sp., which cause obstacles to the continuous cropping of lily. After intercropping, the relative abundance of these fungi in the maize roots decreased; but in the lily roots, it increased.
The total relative abundance of soil bacteria in the roots of lily and maize was reduced after the two crops were interplanted, and the highest abundance of bacteria was viridiflava. The relative abundance of viridiflava in the rhizosphere soil after interplanting was higher than that in the monoculturing system. Viridiflava is a species of bacteria that causes disease in the cultivation of melon and fruit (Zhang et al., 2013 ); whether it will be the cause of lily disease is unknown. Some of the higher abundances of bacteria, such as coli, gnavus, and citroniae are intestinal bacteria, which may be because irrigation brings a large number of intestinal bacteria into the root soil. Bacillus subtilis and streptomyces can inhibit lily fusarium wilt pathogens (Han, 2010); an active substance produced by Bacillus inhibits the growth of plant pathogens (Osouli and Afsharmanesh, 2016). Bacillus sp. and streptomyces sp. had a certain relative abundance in the bacterial composition measured by 16S rDNA. However, the relative abundance of the two types of microorganisms in the interplanting system decreased, as compared to the monoculturing system.
Through the OTU clustering analysis, there were some microbes in the interplanting system that did not exist in the monoculturing system in the rhizosphere soil of Lanzhou lily. The fungi are Pleosporales sp., Scleroderma sp., and Chaetomium sp.. The bacteria are Clostridium sp., Fimbriimonas sp., Coprococcus sp., and Flavobacterium sp.. Cao et al. extracted secondary metabolites from Pleosporales with strong resistance to agricultural pathogenic fungi (Cao et al., 2016 ). Chaetomium sp. is an important resource for a class of fungi as an effective biocontrol agent against plant pathogens (Zhang, 2013). Clostridium sp., Fimbriimonas sp., Coprococcus sp., and Flavobacterium sp. are all pathogenic microorganisms that are harmful to plants. Among the fungi from lily rhizosphere soil in the monoculturing system, Verticillium sp. produces a verticillium wilt disease; fungi Zygopleurage sp. and bacteria Allobaculum sp. are microorganisms from the intestinal tract.
In summary, after interplanting, the relative abundance of some microorganisms increased the harmful fungi of lily rhizosphere soil; and the relative abundance of beneficial bacteria decreased. This situation is not conducive to easing the obstacles to the continuous cropping of lily. By OTU clustering, some beneficial fungi were present in the soil of lily with interplanting that were not in the soil in a lily monoculture. At the same time, some soil-borne pathogens and intestinal pathogens were not detected with interplanting. These findings indicate that the changes in the microbial structure and composition of the roots of lily did not have a specific set of rules, and the change was extremely complicated.
In terms of diversity, there was no significant difference between the different patterns of planting. The richness index Chao1, the uniformity index (ACE), and the diversity indexes Shannon and Simpson of the roots of the lily roots increased after the intercropping (Table 3). This change of diversity indexes indicates that the number and diversity of fungal species in the lily rhizosphere soil increased. However, the index of the lily rhizosphere soil became smaller after intercropping (Table 5). This finding indicates that, after intercropping lily and maize, the richness and diversity of the lily rhizosphere soil bacteria decreased. The changes in the diversity of fungi and bacteria in the roots of lily were different from most changes to plants' rhizosphere soil microbials. The effect of interplanting on plant environmental factors may increase the diversity of the bacterial community structure (Zhou et al., 2011 ). Plant diversity can increase the diversity of soil microbes (Stephan et al., 2000 ). The results showed that the soil microbial diversity decreased after interplanting, which is inconsistent with previous results. We suggest reasons for this result: First, it may be related to a difference for the species, with this being the first study of soil microbes in the Lanzhou lily-and-maize intercropping system. Therefore, there is no direct reference to the trend of changes in microbes. The second reason is possibly related to the sampling period; the microbial community structure of the rhizosphere soil at the different growth stages of kiwifruit is different (Fu et al., 2015 ).
In addition to measuring the changes of microbials in soils of different cropping patterns, we used GC–MS technique to determine the root exudates of different cropping patterns. The results show that interplanting reduced the composition of the lily root exudations, while the number of species of organic acids, phenolic acids, sugars, amino acids, and other substances decreased. The relative content of phenolic acids, sugars, and amino acids decreased; but the relative content of organic acids increased (Figure 8). Root exudates are the carbon source and energy for soil microbes. The decrease of relative abundance of soil microbes in the lily roots is probably related to the decrease of the species of root exudates. Phenolic compounds inhibit the growth of soil microbes, while influencing the amount and activity of microorganisms in rhizosphere soil (Xue et al., 2005 ). The changes of amino acid and total sugar content in root exudates are beneficial to the occurrence of fusarium wilt disease (Gao and Zhang, 1998). Therefore, after intercropping lily and maize, the reduction of phenolic substances is conducive to reducing the autotoxicity of Lilium davidii var. unicolor. The change of the relative content of sugars and amino acids promotes the growth of pathogens of Lilium wilt pathogen.
5 ConclusionAfter interplanting lily and maize, we found that the structure and diversity of the soil microbes in lily's rhizosphere soils were changed, which also affected the content of the lily root exudates. There were no differences in the composition of the soil microbes in the lily roots at the phylum level, and the relative abundance of soil fungi and bacteria in the lily roots decreased. However, the relative abundance of Fusarium sp. and other lily pathogens and the pathogenic bacteria viridiflava increased in the lily's root soil. At the same time, the relative abundance of beneficial bacteria such as Bacillus subtilis and Streptomyces sp. strains with inhibitory effects on Fusarium sp. was reduced after interplanting. However, there were fungi such as Pleosporales, which are beneficial to plant growth, that do not exist in the monoculturing system. Meanwhile, some of the disease bacteria present at the time of monoculturing were not detected in the lily's rhizosphere soil after intercropping; through interplanting, the diversity of fungi in the roots of lily was higher than that in monoculturing; and the diversity of bacteria was lower than that in monoculturing. The reason for this result may be related to the specificity of the species and may also be related to the sampling period. The decrease in the composition of root exudates may be the reason for the decrease in the relative abundance of rhizosphere soil microbes. The relative content of phenolic compounds in the root exudates can alleviate the autotoxicity of the continuous cropping of lily, but the change of relative contents of amino acids and carbohydrates may promote the occurrence of fusarium wilt in Lanzhou lily. In summary, after intercropping lily and maize, root exudates and soil microbial changes are very complex. There are also advantages and disadvantages to optimizing the cultivation of Lanzhou lily. And the interaction between root exudates and rhizosphere soil microbes is also very complex. Therefore, we expect to optimize the cultivation of Lanzhou lily by using interplanting techniques to mitigate against the obstacles to continuous cropping of Lanzhou lily, which also requires much research about the mechanics that control the growing conditions.
Acknowledgments:This study was funded by Lanzhou Branch of the Chinese Academy of Sciences institutional cooperation program (2BY52BI61) and the Key program of Chinese Academy of Sciences (22Y622AM1). We thank technician Qiuming He for helping us collect samples.
Asao T, Hasegawa K, Sueda Y, et al. 2003. Autotoxicity of root exudates from taro. Scientia Horticulturae, 97(3–4): 389-396. DOI:10.1016/S0304-4238(02)00197-8 |
Benayas JMR, Newton AC, Diaz A, et al. 2009. Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science, 325(5944): 1121-1124. DOI:10.1126/science.1172460 |
Bertin C, Yang XH, Weston LA. 2003. The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil, 256(1): 67-83. DOI:10.1023/A:1026290508166 |
Braun PG. 1991. The combination of Cylindrocarpon lucidum and Pythium irregulare as a possible cause of apple replant disease in Nova Scotia
. Canadian Journal of Plant Pathology, 13(4): 291-297. DOI:10.1080/07060669109500914 |
Braun PG. 1995. Effects of Cylindrocarpon and Pythium species on apple seedlings and potential role in apple replant disease
. Canadian Journal of Plant Pathology, 17(4): 336-341. DOI:10.1080/07060669509500672 |
Cao F, Zhang BY, Li W, et al. 2016. Bioactive azaphilones from a marine-derived Pleosporales sp
. fungus collected from the Bohai Sea. Chinese Journal of Marine Drugs, 35(3): 6-10. |
Chen LH, Yang XM, Raza W, et al. 2011. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers
. Applied Microbiology and Biotechnology, 89(5): 1653-1663. DOI:10.1007/s00253-010-2948-x |
Fu QX, Gu J, Li YD, et al. 2015. Analyses of microbial biomass and community diversity in kiwifruit orchard soils of different planting ages. Acta Ecologica Sinica, 35(3): 22-28. DOI:10.1016/j.chnaes.2015.04.002 |
Gao ZQ, Zhang SX. 1998. Continuous cropping obstacle and rhizospheric microecology I. Root exudates and their ecological effects. Chinese Journal of Applied Ecology, 9(5): 549-554. |
Gullino ML, Daughtrey ML, Garibaldi A, et al. 2015. Fusarium wilts of ornamental crops and their management. Crop Protection, 73: 50-59. DOI:10.1016/j.cropro.2015.01.003 |
Haichar FEZ, Santaella C, Heulin T, et al. 2014. Root exudates mediated interactions belowground. Soil Biology & Biochemistry, 77(7): 69-80. DOI:10.1016/j.soilbio.2014.06.017 |
Han L, 2010. Inhibitive and prevent effects of biocontrol agents and garlic bulb crude extracts on lily fusarium wilt. Yangling: Northwest A&F University, pp. 5–6.
|
Hou YH, 2012. The influences of fertilization and arbuscular mycorrhizal fungi on the growth of Lilium davidii var.unicolor. Lanzhou: Lanzhou University, pp. 8–9.
|
Iannucci A, Fragasso M, Platani C, et al. 2013. Plant growth and phenolic compounds in the rhizosphere soil of wild oat (Avena fatua L.)
. Frontiers in Plant Science, 4: 509. DOI:10.3389/fpls.2013.00509 |
Jaffee BA, Abawi GS, Mai WF. 1982. Fungi associated with roots of apple seedlings grown in soil from an apple replant site. Plant Disease, 66(10): 942-944. |
Kind T, Wohlgemuth G, Lee DY, et al. 2009. FiehnLib-mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Analytical Chemistry, 81(24): 10038-10048. DOI:10.1021/ac9019522 |
Kourtev PS, Ehrenfeld JG, Häggblom M. 2003. Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biology and Biochemistry, 35(7): 895-905. DOI:10.1016/S0038-0717(03)00120-2 |
Ma JY, Zhao XL, Zhang J, et al. 2005. Progress in research of Lilium davidii var. unicolor
. Journal of Tarim University, 17(4): 53-56, 76. |
Osouli S, Afsharmanesh H. 2016. To evaluate the effects of secondary metabolites produced by Bacillus subtilis mutant M419 against Papilio demoleus L. and Aspergillus flavus
. Acta Ecologica Sinica, 36(6): 492-496. DOI:10.1016/j.chnaes.2016.08.002 |
Ren LX, Su SM, Yang XM, et al. 2008. Intercropping with aerobic rice suppressed Fusarium wilt in watermelon
. Soil Biology and Biochemistry, 40(3): 834-844. DOI:10.1016/j.soilbio.2007.11.003 |
Sanguin H, Sarniguet A, Gazengel K, et al. 2009. Rhizosphere bacterial communities associated with disease suppressiveness stages of take-all decline in wheat monoculture. New Phytologist, 184(3): 694-707. DOI:10.1111/j.1469-8137.2009.03010.x |
Seastedt TR, Callaway RM, Pollock JL, et al. 2008. Allelopathy and plant invasions: traditional, congeneric, and bio-geographical approaches. Biological Invasions, 10(6): 875-890. DOI:10.1007/s10530-008-9239-9 |
Sewell GWF. 1981. Effects of Pythium species on the growth of apple and their possible causal role in apple replant disease
. Annals of Applied Biology, 97(1): 31-42. DOI:10.1111/j.1744-7348.1981.tb02992.x |
Stephan A, Meyer AH, Schmid B. 2000. Plant diversity affects culturable soil bacteria in experimental grassland communities. Journal of Ecology, 88(6): 988-998. DOI:10.1046/j.1365-2745.2000.00510.x |
Su BY, Chen SB, Li YG, et al. 2013. Intercropping enhances the farmland ecosystem services. Acta Ecological Sinica, 33(14): 4504-4514. DOI:10.5846/stxb201204200574 |
Sutton TB, Hayne DW, Sullivan WT, et al. 1981. Causes of apple tree death in Henderson County, North Carolina. Plant Diseases, 65: 330-332. |
Taylor JB, Wallace BD. 1970. Root canker in stone fruit caused by the fungus Peniophora sacrata
. Orchardist of New Zealand, 43: 263-265. |
Thevathasan NV, Gordon AM. 2004. Ecology of tree intercropping systems in the North temperate region: experiences from southern Ontario, Canada. Agroforestry Systems, 61(1–3): 257-268. DOI:10.1023/B:AGFO.0000029003.00933.6d |
Utkhede RS, Vrain TC, Yorston JM. 1992. Effects of nematodes, fungi and bacteria on the growth of young apple trees grown in apple replant disease soil. Plant and Soil, 139(1): 1-6. DOI:10.1007/BF00012835 |
Wu LK, Lin XM, Lin WX, et al. 2014. Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chinese Journal of Plant Ecology, 38(3): 298-310. DOI:10.3724/SP.J.1258.2014.00027 |
Wu ZJ, Xie ZK, Yang L, et al. 2015. Identification of autotoxins from root exudates of Lanzhou lily (Lilium davidii var. unicolor)
. Allelopathy Journal, 35(1): 35-48. |
Xue CY, Wu FZ, Wang HC, et al. 2005. The summary of reciprocity between phenolic acids and soil microorganism. Heilongjiang Agricultural Sciences, (3): 45-47. DOI:10.3969/j.issn.1002-2767.2005.03.015 |
Zhang F, 2013. Isolation, screening of Chaetomium spp. and preliminary study on biocontrol mechanisms. Tai'an: Shandong Agricultural University.
|
Zhang L, Tian Q, Liu FQ, et al. 2013. Six seedbome bacterial pathogens screening with chromogenic DNA macroarray in melon crops. Journal of Agricultural Biotechnology, 21(1): 120-126. DOI:10.3969/j.issn.1674-7968.2013.01.015 |
Zhou XG, Yu GB, Wu FZ. 2011. Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. European Journal of Soil Biology, 47(5): 279-287. DOI:10.1016/j.ejsobi.2011.07.001 |
 2018, Vol. 10
2018, Vol. 10
