2. Department of Chemistry, Villanova University, Villanova, PA 19085, USA;
3. Department of Chemistry and Biochemistry, Old Dominion University, Norfolk, VA 23529, USA
Dissolved organic matter (DOM) is a complex mixture of thousands of organic compounds and is a ubiquitous component of terrestrial and marine aquatic samples. The chemical complexity of DOM makes it difficult to characterize; and an improved understanding of the molecular composition of DOM is needed to allow the sources, chemical transformation processes, and environmental impacts of DOM to be evaluated (as described by Nebbioso and Piccolo, 2013). DOM can be stored in ice-core samples for hundreds or thousands of years, and elucidation of the chemical composition can help reconstruct information on past climates and environments (Grannas et al., 2006 ). Many studies of ice cores have focused on a specific DOM fraction (Legrand et al., 2007 ), such as specific low-molecular-weight organic compounds (Legrand et al., 2003 ; Kawamura et al., 2012 ), which provide molecular clues to the environmental conditions when the DOM was deposited. However, this approach has often led to an incomplete picture of the DOM composition because a large fraction of DOM cannot be identified using conventional approaches.
Electrospray ionization (ESI) combined with ultra-high resolution Fourier transform ion cyclotron resonance mass spectrometry (ESI-FT-ICR-MS) has recently and effectively been used to characterize the complex molecular composition of DOM because it allows exact molecular weights and formulae of thousands of individual organic molecules to be assigned. Grannas et al. (2006) first used ESI-FT-ICR-MS to analyze DOM in two ice-core samples that had been deposited in approximately 1300 A.D. and 1950 A.D. in Franz Josef Land, Russia. They found that molecules containing sulfur were more abundant in the modern ice than in the older ice and attributed this increase in S incorporation to atmospheric processes occurring in a more SOx-rich environment. Marsh et al. (2013) used a nano-ESI-FT-ICR-MS method to analyze small volumes (approximately 90 mL) of meltwater from two ice-core samples, deposited in 1360 A.D. and 1660 A.D., from Greenland. They used two different solid-phase extraction resins (C18 and PPL) to extract and concentrate the DOM and contrasted the molecular composition of the DOM in these two extracts. The results show that PPL had higher extraction efficiency for organic matter (such as CHO components). The DOM in surface snow and ice samples from several sites has been characterized by ESI-FTICR-MS in different studies; and improvements to analytical methods and molecular information about DOM were obtained, such as the importance of DOM sources from terrestrial and microbial input (Bhatia et al., 2010 ; Singer et al., 2012 ; Stubbins et al., 2012 ; Antony et al., 2014 ; Lawson et al., 2014 ).
Ice cores collected from the Greenland ice sheet can contain ice from more than 105 ka ago, which includes ice deposited over a number of glacial–interglacial cycles. DOM in ice cores deposited under different climatic conditions may reflect differences in the provenance of the organic matter (OM) and differences in the chemical, abiotic, and biotic processes that affected the OM under the various conditions, as illustrated from inorganic components (Wolff et al., 2010 ). Warm and wet interglacial climates, such as during the Holocene, favoured increased plant cover and precipitation; and marine ecosystems would also have been active, meaning that a great deal of phytoplankton metabolism would have occurred. During glacial periods, especially the Last Glacial Maximum (LGM), due to lowering the sea level and exposing continental shelves, ecosystems may have switched to dramatically different states (Farrera et al., 1999 ; Otto-Bliesner et al., 2006 ). It is therefore possible that the proportions of aquatic and terrestrial OM in the environment varied as the climate changed. In addition, biological DOM can differ between marine and terrestrial sources; the photochemical processes occurring during the transport of DOM and after deposition may also differ under different climatic conditions. Because of these differences, the molecular composition of DOM samples from ice cores may allow compounds affected by changes in the climate to be "fingerprinted," providing some insight into the factors that controlled the types and reactivity of the DOM compounds.
In this study, we used ESI-FT-ICR-MS to examine the DOM preserved in two Greenland ice-core samples, comparing glacial and interglacial time periods. Although this work was limited to only two samples, our aim was to give a preliminary assessment of DOM composition in glacial/interglacial ice, which to date has not been assessed in the literature.
2 Methods 2.1 Sample collection and pre-analysis processingThe ice core in this study was collected as part of the North Greenland Eemian Ice Drilling (NEEM) project, and details of the core can be found at http://neem.dk. Briefly, the NEEM ice core, which was 2,540-m long, was taken from the Northwest Greenland ice sheet at 77.45°N, 51.06°W. The core was drilled between 2008 and 2012; and the objective was to retrieve ice from the previous interglacial, the Eemian, which ended about 115,000 years ago. The ice core was cut in the field, and one-quarter of a 20-cm-long subsection of each 10-m section of the ice core was sent to China (one of the members of the NEEM project) for trace-metal analysis using inductively coupled plasma mass spectrometry (ICP-MS). Two melted samples from the core were produced during the ICP-MS measurements by combining parts of several samples into 250-mL Duran glass containers (Schott, Mainz, Germany). The glassware was acid-washed following the procedure used throughout the ICP-MS analysis. The sample details, including the date and depth of each sample, are presented in Table 1. The samples were aged, from the age–depth relationship, to 1692–4379 B.P. before present (Holocene sample: HS) and 21627–24804 B.P. (LGM sample: LS), respectively. The samples were transported to the laboratory, where they were prepared and analyzed in a cooling box at <0 °C.
|
|
Table 1 Concentrations and quality indicators (average for the entire sample) for the DOM isolated from samples from the NEEM ice core, Greenland, and characterized by negative ESI-FT-ICR-MS |
DOM samples were desalted and concentrated using an established procedure for PPL solid-phase extraction cartridges (Dittmar et al., 2008 ), and the desalted samples were eluted into LC-MS-grade methanol. Each sample was directly infused into an Apollo II ESI ion source operated in the negative ion mode at a flow rate of 2 μL/min on a Bruker Daltonics 12 Tesla Apex Qe FT-ICR-MS (Bruker, Billerica, MA, USA), housed at the College of Sciences Major Instrumentation Cluster at the Old Dominion University. Spray voltages were optimized for each sample. Ions were accumulated in the hexapole for 3.0 seconds before transfer into the ICR cell, where exactly 300 transients were co-added.
2.3 Data processingEach mass spectrum was externally calibrated using a polyethylene glycol (PEG) standard. Internal calibration of the acquired mass spectra was achieved using numerous CH2 homologous series identified by Kendrick mass defect (KMD) analysis that spanned the m/z range of 200–700. Peaks present in the blank, or those identified as a13C analog or a salt peak (due to a high mass defect) were removed from the peak list prior to molecular-formula assignment. Mass lists (of peaks with a signal-to-noise [S/N] ratio exceeding 4) were processed via a molecular-formula calculator to determine all possible molecular formulae (12C5-801H5-10016O1-3014N0-532S0-531P0-2) for each m/z value, within a maximum error of 1.0 ppm. The formula rules applied by Stubbins et al. (2010) were followed to ensure that chemically possible molecular formulae were assigned to the peaks in each mass spectrum. At higher m/z values, unambiguous formula assignment was not possible in some cases due to several possible molecular formulae existing for the same m/z value. To assist in duplicate removal, the "formula extension" approach (as described in Kujawinski and Behn, 2006) was utilized with homologous series of CH2, CHO, and CH2O groups to determine the appropriate formula. Although it is possible, and even likely, that multiple formulae exist for the same m/z, the formula-extension approach offers support for the presence of certain formulae over formulae not part of a Kendrick mass defects (KMD) series. Peaks that could not be assigned an unambiguous formula were removed from further consideration. Approximately 70% of the peaks were assigned molecular formulae using these constraints.
The assigned molecular formulae were examined using the van Krevelen diagram, double-bond equivalents (DBEs), KMDs, and aromatic indices (AImod). The O/C and H/C ratios were calculated by dividing the number of O and H atoms, respectively, by the number of C atoms in a formula. DBE analysis was used to determine the number of rings and double bonds in a molecule. The DBE was calculated using Equation (1),
| ${\rm{DBE}} = 1 + c - h/2 + n/2 + p/2$ | (1) |
where c, h, n, and p are the numbers of C, H, N, and P atoms, respectively, in the formula.
The KMD representation of high-resolution mass spectral data can be used to search for potential oligomeric units (Hughey et al., 2001 ). The Kendrick mass (KM) and KMD for CH2 series were calculated using Equations (2) and (3),
| ${\rm{KM}} = {\rm{observed}}\;{\rm{mass}} \times 14/14.01565$ | (2) |
| ${\rm{KMD}} = {\rm{NM}}-{\rm{KM}}$ | (3) |
where 14 is the nominal mass (NM) of CH2, 14.01565 is the exact mass of CH2, and NM is KM rounded to the nearest integer. A homologous series of compounds differing by only the number of base units form a horizontal line in a plot of KMD against KM.
AImod is a measure of the probable aromaticity of a molecule, assuming that half the O atoms are double-bonded and half have only σ bonds (Koch and Dittmar, 2006). AImod was calculated using Equation (4),
| ${\rm{A}}{{\rm{I}}_{{\rm{mod}}}} = \left( {1 + c - 0.5o - s - 0.5h} \right)/\left( {c - 0.5o - s - n - p} \right) $ | (4) |
where c, o, s, h, and p are the number of C, O, S, H, N, and P atoms in the formula. AImod ranges from 0 for a purely aliphatic compound to 1, with higher values being found for compounds with more double bonds and that are more aromatic.
The formulae were plotted on the van Krevelen diagram; then, each was categorized using the stoichiometry of its molecular formula. The groups relevant to this study were identified using AImod and H/C cutoffs, which were AImod ≥0.67 for combustion-derived condensed aromatics, 0.67> AImod ≥0.5 for highly unsaturated aromatic compounds, AImod <0.5 and H/C ≤1.5 for lignin-like compounds and carboxylic-rich alicyclic molecule (Lignin/CRAM) compounds, and 2.0> H/C >1.5 for aliphatic compounds ( Koch and Dittmar, 2006; Hockaday et al., 2009 ).
3 Results and discussionMolecular formulae with the form CcHhOoNnSsPp were assigned to the negative-ion ultrahigh-resolution FT-ICR mass spectra of the DOM components extracted from the ice-core meltwater samples. Overall, 80% of the total ion current mass spectra of the samples was assigned. The number of peaks assigned molecular formulae was 919 and 149, for HS and LS, respectively, excluding contributions from compounds containing 13C isotopes and peaks with high mass defects, indicative of salts.
3.1 General FT-ICR-MS characteristicsReconstructed mass spectra for the monoisotopic molecular formulae assigned to the samples after blank subtraction are shown in Figure 1. Seven subgroups of the molecular formulae were identified, based on the elements in the formulae. These subgroups were compounds containing only C, H, and O (CHO); compounds containing C, H, O, and N (CHON); compounds containing C, H, O, and S (CHOS); compounds containing C, H, O, and P (CHOP); compounds containing C, H, O, P, and N (CHOPN); compounds containing C, H, O, S, and P (CHOSP); and compounds containing C, H, O, N, and S (CHONS). The most abundant composition in both samples was CHO (contributing 50% of all formulae); and the contributions of the other subgroups decreased in the order of CHOS (13%–19%), CHOP (13%–22%), CHONS (6%–9%), CHON (3%–8%), CHOPN (0%–3%), and CHOSP (0%–3%). The compositions according to these subgroups were very similar to compositions found in snow samples from Mendenhall Glacier, southeastern Alaska (Stubbins et al., 2012 ), but different from compositions found in aerosol and fog water, in which CHON formulae have sometimes been found to be dominant (Zhao et al., 2013 ; Wozniak et al., 2014 ).
Although CHO-containing formulae comprised the majority of the identified OM, a significant portion of the OM contained heteroatoms of varying combinations of S, N, and P. Wozniak et al. (2014) found that water-soluble OM in aerosol particulates collected over a marine environment had primary marine biological sources and was also rich in compounds containing N and P. Lawson et al. (2014) employed FT-ICR-MS analysis on Greenland Ice Sheet runoff samples and found a high proportion of N-rich DOM. The requirements for N and P are higher for marine phytoplankton and bacteria than for terrestrial vegetation; and dissolved organic N and P have long been known to be enriched in the sea-surface micro-layer, where surface-active OM accumulates after being entrained by bubbles and brought to the surface (Miyazaki et al., 2011 ; Buchan et al., 2014 ). The abundance of compounds containing N and P in these Greenland ice-core samples likely indicates that the DOM primarily had a marine biological source.
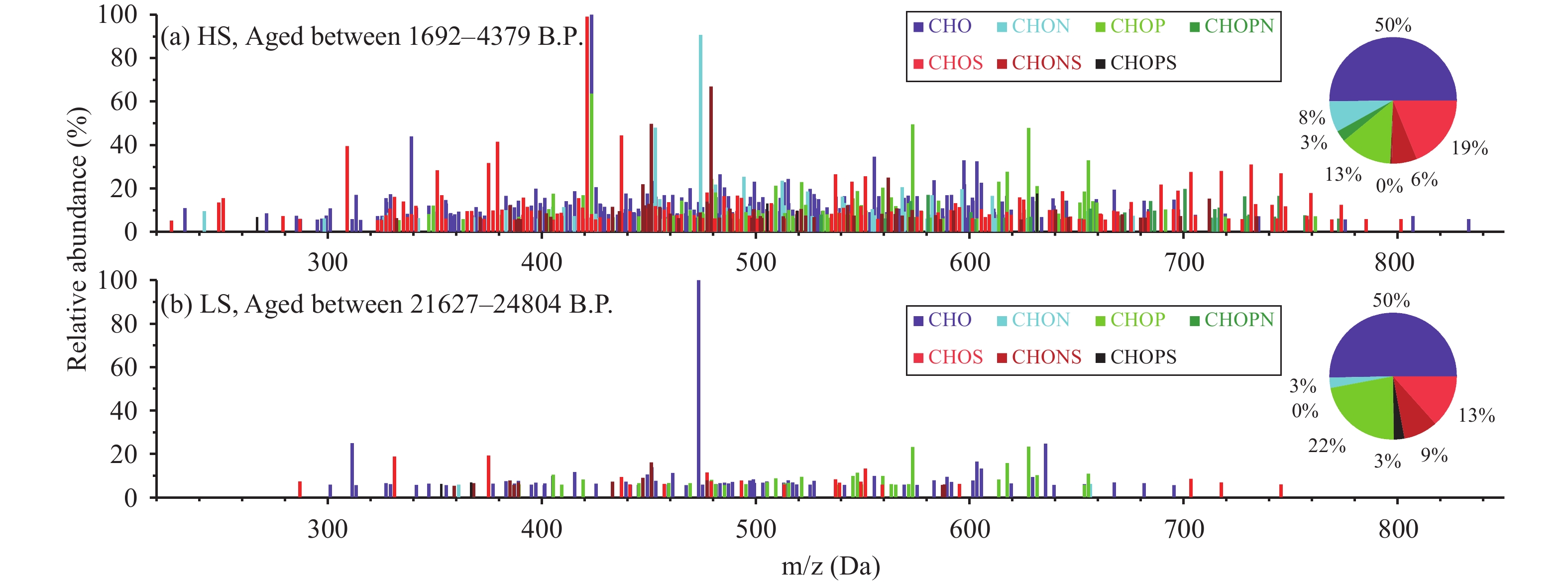
|
Figure 1 Negative ESI mass spectra for the DOM in the two NEEM ice-core samples (HS and LS). The pie charts show the contributions of the different compound classes to the totals in the different samples |
Biological and geochemical classes of organic compounds in DOM can be identified from their distinct elemental ratios using van Krevelen analysis (Grannas et al., 2006 ; Stubbins et al., 2012 ). The contributions of different molecular-formula classes to the aliphatic, Lignin/CRAM, aromatic, and condensed aromatic compounds are shown in Figures 2a and 2b. Overall, HS and LS had similar molecular signatures, as determined from the distributions of formulae in the van Krevelen space (Figure 2b), which indicates that the dominant sources of DOM for these two samples were likely similar.
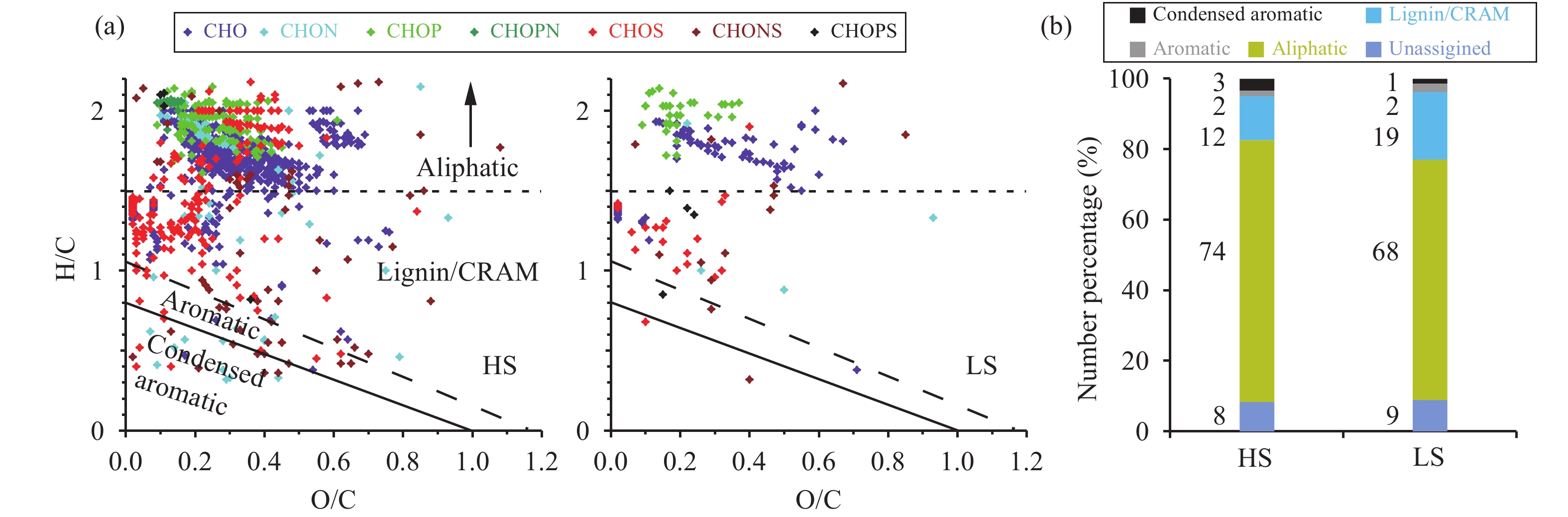
|
Figure 2 (a) Van Krevelen plots for Holocene sample (HS) and LGM sample (LS) and (b) a bar chart showing the contributions of each structural class (determined using AImod and H/C ratio) to the total DOM |
The main molecular structures in the samples were aliphatic (68%–74%), the next most common was Lignin/CRAM (12%–19%), and there was a small number of aromatic and condensed aromatic compounds (2%–3%). These contributions were similar to those previously found in snowpack and glacier outflow samples from southeast Alaska (Stubbins et al., 2012 ). A large contribution of aliphatic compounds has also been found in samples collected far from the coast in Antarctica (Antony et al., 2014 ), where in situ microbial sources have little influence.
The average O/C ratios for both samples were relatively low, 0.29 for HS and 0.27 for LS, respectively, indicating less oxidized aerosols were dominant contributors to DOM. The average O/C ratio observed from aged aerosol in remote areas can be up to 1.0, using aerosol mass spectrometry (Xu et al., 2015 ); and less than 0.3 for less oxidized aerosol or rainwater samples (Altieri et al., 2009 ; Wozniak et al., 2014 ). The O/C ratios in the DOM of snowpack and glacier outflow in the Mendenhall Glacier in southeast Alaska are also close to 0.3 (Stubbins et al., 2012 ), less than 0.5 in most of the formulae in the Holocene DOM of one Greenland ice core (Marsh et al., 2013 ) and less than 0.4 for the ice core DOM in Franz Josef Land (Grannas et al., 2006 ).
Compounds containing N and P were mostly aliphatic, with high H/C ratios (>1.7) and low O/C ratios (<0.4), suggesting the feature of less being oxidized.Wozniak et al. (2014) found that the primary sources of aliphatic CHON compounds in marine aerosol DOM were marine and biological. Most of the CHO compounds were also aliphatic, with higher O/C ratios and lower H/C ratios than the compounds containing N and P. The higher O/C ratios of CHO compounds suggest that a portion of the aerosols were oxidized during transport before deposition (Wozniak et al., 2014 ). HS was richer in aliphatic S compounds than LS, which may have had primary and/or secondary aerosol sources. Aliphatic compounds rich in S are prevalent in the water-soluble organic carbon fractions of aerosols and probably come from the aqueous oxidation of biogenic volatile organic compounds during the generation of secondary organic aerosols, i.e., organosulfates (Surratt et al., 2007 ). Organic compounds containing S, such as dimethyl sulfide and dimethylsulfoniopropionate, can also be emitted from marine environments (Andreae et al., 2003 ) and influence S-containing DOM. The primary and/or secondary S aerosols can be further distinguished by DBE distribution (Section 3.4). The relatively low abundance of aliphatic S in LS probably means that fresh and aged S aerosol sources were relatively weak during the LGM.
HS contained a larger number of condensed aromatic formulae than LS. The aromatic compounds mostly contained N and S. Condensed aromatics can be formed only through the heating of OM or through intense photochemical activity (Yee et al., 2013 ; Chen et al., 2014 ), so their presence indicates that HS may contain combusted OM or that the DOM had undergone significant photochemical reactions during transport. The results therefore suggest that terrestrial sources (such as forest fires) of DOM were important in HS.
3.3 KMD analysis of DOM componentsThe homologous groups and homologs within each individual group can be identified using a KMD plot (Mopper et al., 2007 ). The KMD plots for these two samples are shown in Figures 3a and 3b; and plots for the different compound classes in HS and LS are shown in Figures 3c, 3d, 3e, and 3f. Points forming horizonta, l line corresponds to families of species with formulae CxHyOz(CH2)n, with fixed O-atom contents (x, y, and z are fixed; and n is a variable) and different numbers of CH2 groups. A large number of CH2 homologous series were found in the CHO, CHOS, CHNO, CHOP, and CHOPN compound classes in HS. The KMD values of these relatively abundant compounds tended to increase as the molecular mass increased, suggesting that increases in the molecular masses were probably caused by increasing numbers of heteroatoms (especially O and S) in the compounds. Overall, no significant families are oberserved in LS due to the smaller number of identified formulae. The most related formulae in the series in CHON compound of HS was the N3O7 family, and the next longest were the NO3 and N2O8 families (Figure 3). On average, more than 98% of the CHON compounds in the sample had O/N ratios >3. The high O/N ratio is consistent with formation of organic nitrate, likely due to the known chemistry of nitrate radicals identified in ambient aerosols ( Ng et al., 2008 ).

|
Figure 3 Kendrick mass plots of the unique neutral molecular formulae, normalized to CH2 for HS and LS for (a) total HS formulae, (b) total LS formulae, (c) CHO formulae, (d) CHON formulae, (e) CHOP formulae, and (f) CHOS formulae |
The DBEs of DOM molecules also allow assessment of the intensity of different DOM sources and the atmospheric conditions to which the aerosol was exposed (Bateman et al., 2011 ). The CHO compound DBEs with respect to C of these two samples are shown in Figures 4a and 4b. Most (>90%) of the CHO compounds in both samples had DBEs of <10, and the compounds with high relative abundances had DBEs of 2~7. The most abundant DBEs were 6 in HS and 4 in LS; and the average DBEs were 5.7 and 5.9, respectively. The compounds with the highest relative abundance in both samples had 10~40 C atoms. Compounds with >40 C atoms were also found in HS. The DBEs of CHO compound in HS and LS were similar to those in modern aerosols and fog water (5–7) (Mazzoleni et al., 2010 ; Zhao et al., 2013 ); but the average CHO compound O/C ratios (0.31 and 0.29 for HS and LS, respectively) were lower than in aerosols and fog water (0.4–0.7), suggesting that the CHO compounds in the ice-core samples were less oxidized.
Higher average DBEs were found for the CHOS compounds in both samples (10 and 14 for HS and LS, respectively), suggesting unsaturated CHOS compounds in these two samples. Two subgroups of CHOS molecular formulae in HS were highlighted, based on H/C value (Figure 4c). The CHOS compounds in subgroup 1 with H/C ≥1.5 are relatively saturated, with DBE values of 0–7. The subgroup 2 CHOS compounds were very unsaturated, with DBEs of 10–20. The O/C ratio was clearly higher in subgroup 1 (0.30) than in subgroup 2 (0.15). The high O content and low DBEs of the CHOS compounds in subgroup 1 suggest these compounds could be organosulfates. However, a large number of S-containing compounds containing reduced forms of S, indicated by the low O content in the molecular formulae, have rarely been reported (Vairavamurthy and Mopper, 1989; Willoughby et al., 2014 ). These compounds may have come from fresh emissions rather than being aged products. The average DBEs for CHOP and CHON compounds in HS and LS are all less than 10, similar to DBEs for the CHO compounds.
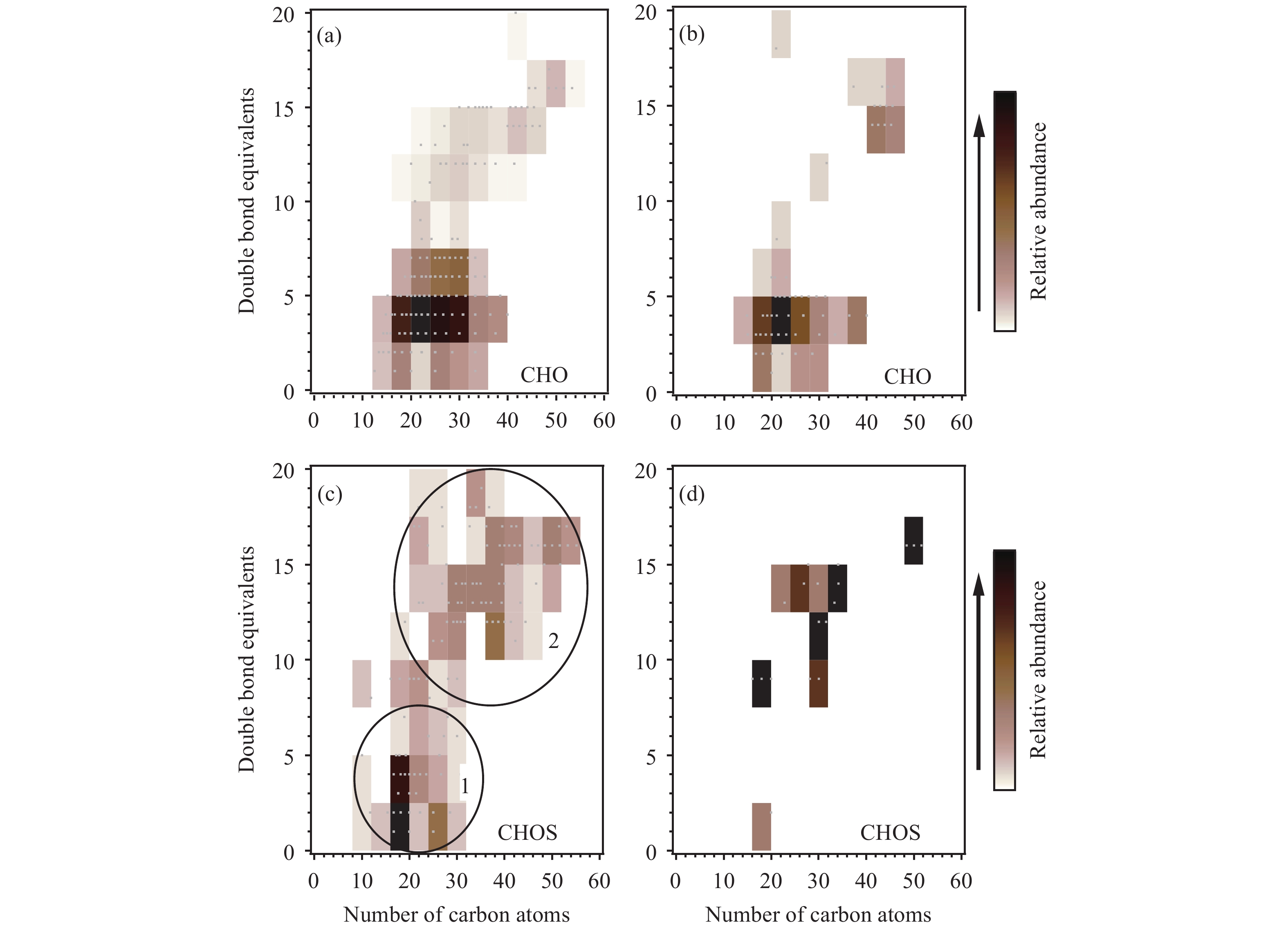
|
Figure 4 Isoabundance plot of the double bond equivalents (DBEs) against the number of C atoms in the molecular formulae containing C, H, and O for (a) HS and (b) LS and for the molecular formulae containing C, H, O, and S for (c) HS and (d) LS. The two subgroups shown for HS in (c) were separated using their H/C ratios (H/C <1.5 for subgroup 1 and H/C ≥1.5 for subgroup 2) |
We performed the first detailed analysis of DOM in LGM and Holocene ice-core samples from Greenland using FT-ICR-MS. The molecular characteristics identified in these samples suggest the present of compounds derived from primarily terrestrial and marine sources and that some secondary processing occurred in the atmosphere. The compounds containing N and P are consistent with marine primary sources. Condensed C compounds in the DOM suggested contributions from terrestrial combustion sources. The presence of organosulfates and organonitrates suggests secondary reactions in the atmosphere influenced this DOM. The concentration and photochemical processes of DOM were higher and stronger during the warm period (HS) than during the cold period (LS); but the chemical compositions are very similar, suggesting the common DOM sources.
One weakness of this study is that only two samples are used, which means the results could not be representitive; however, the availability of such samples is severly limited. A large sample size is desirable and possible in the future study from multiple ice cores at different sites. In addition, the extraction of DOM using PPL resin can impart loss of exceptionally hydrophillic compounds and small molecules. However, the losses are expected to have minimal negative impact because compounds with m/z less than 200 are not efficiently detected by FT-ICR-MS. PPL is expected to retain 60%–75% of OM (Dittmar et al., 2008 ; Stubbins et al., 2012 ). Finally, FT-ICR-MS is providing a picture of only polar compounds ionizable by negative ESI between 200–1,000 m/z. Despite bias, the amount of molecular information provided is still very useful. A combination of a suite of instruments (such as nuclear magnetic resonance spectroscopy, NMR) in future studies is needed to give an even broader characterization of the OM.
Our results could also help inform future ice-core studies in Northwest Greenland. The chemical characteristics of the DOM showed variations in the importance of marine and terrestrial sources—for example, significant terrestrial sources for HS; and that the high-resolution analysis of ice-core samples is required to allow marine and terrestrial impacts on the aerosols transported to Greenland to be further distinguished. The contributions of compounds formed through atmospheric reactions suggest that atmospheric conditions have strong impacts on the chemical compositions of aerosols, and this may be of interest when interpreting the results of DOM in modern ice cores that contain anthropogenic aerosols. Modern aerosols in Greenland are affected by the sources described above and also by anthropogenic organic compounds that have been transported in the atmosphere over long distances before being deposited. Our results should motivate an expansion of studies of atmospheric DOM to modern times and the determination of the potential for secondary anthropogenic compounds being deposited in remote regions.
Acknowledgments:This research was supported by grants from the Hundred Talents Program of the Chinese Academy of Sciences, the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (NSFC) (41425003, 41121001), the Ministry of Science and Technology of China (MoST, 2013CBA01804), and the Scientific Research Foundation of the Key Laboratory of Cryospheric Sciences (SKLCS-ZZ-2017-01).
Altieri KE, Turpin BJ, Seitzinger SP. 2009. Oligomers, organosulfates, and nitrooxy organosulfates in rainwater identified by ultra-high resolution electrospray ionization FT-ICR mass spectrometry. Atmospheric Chemistry and Physics, 9(7): 2533-2542. DOI:10.5194/acp-9-2533-2009 |
Andreae MO, Andreae TW, Meyerdierks D, et al. 2003. Marine sulfur cycling and the atmospheric aerosol over the springtime North Atlantic. Chemosphere, 52(8): 1321-1343. DOI:10.1016/S0045-6535(03)00366-7 |
Antony R, Grannas AM, Willoughby AS, et al. 2014. Origin and sources of dissolved organic matter in snow on the East Antarctic Ice Sheet. Environmental Science & Technology, 48(11): 6151-6159. DOI:10.1021/es405246a |
Bateman AP, Nizkorodov SA, Laskin J, et al. 2011. Photolytic processing of secondary organic aerosols dissolved in cloud droplets. Physical Chemistry Chemical Physics, 13(26): 12199-12212. DOI:10.1039/C1CP20526A |
Bhatia MP, Das SB, Longnecker K, et al. 2010. Molecular characterization of dissolved organic matter associated with the Greenland ice sheet. Geochimica et Cosmochimica Acta, 74(13): 3768-3784. DOI:10.1016/j.gca.2010.03.035 |
Buchan A, LeCleir GR, Gulvik CA, et al. 2014. Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nature Reviews Microbiology, 12(10): 686-698. DOI:10.1038/nrmicro3326 |
Chen HM, Abdulla HAN, Sanders RL, et al. 2014. Production of black carbon-like and aliphatic molecules from terrestrial dissolved organic matter in the presence of sunlight and iron. Environmental Science & Technology Letters, 1(10): 399-404. DOI:10.1021/ez5002598 |
Dittmar T, Koch B, Hertkorn N, et al. 2008. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnology and Oceanography: Methods, 6(6): 230-235. DOI:10.4319/lom.2008.6.230 |
Farrera I, Harrison SP, Prentice IC, et al. 1999. Tropical climates at the Last Glacial Maximum: a new synthesis of terrestrial palaeoclimate data. I. Vegetation, lake-levels and geochemistry. Climate Dynamics, 15(11): 823-856. DOI:10.1007/s003820050317 |
Grannas AM, Hockaday WC, Hatcher PG, et al. 2006. New revelations on the nature of organic matter in ice cores. Journal of Geophysical Research: Atmospheres, 111(D4): D04304. DOI:10.1029/2005jd006251 |
Hockaday WC, Purcell JM, Marshall AG, et al. 2009. Electrospray and photoionization mass spectrometry for the characterization of organic matter in natural waters: a qualitative assessment. Limnology and Oceanography: Methods, 7(1): 81-95. DOI:10.4319/lom.2009.7.81 |
Hughey CA, Hendrickson CL, Rodgers RP, et al. 2001. Kendrick mass defect spectrum: a compact visual analysis for ultrahigh-resolution broadband mass spectra. Analytical Chemistry, 73(19): 4676-4681. DOI:10.1021/ac010560w |
Kawamura K, Izawa Y, Mochida M, et al. 2012. Ice core records of biomass burning tracers (levoglucosan and dehydroabietic, vanillic and p-hydroxybenzoic acids) and total organic carbon for past 300 years in the Kamchatka Peninsula, Northeast Asia
. Geochimica et Cosmochimica Acta, 99: 317-329. DOI:10.1016/j.gca.2012.08.006 |
Koch BP, Dittmar T. 2006. From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Communications in Mass Spectrometry, 20(5): 926-932. DOI:10.1002/rcm.2386 |
Kujawinski EB, Behn MD. 2006. Automated analysis of electrospray ionization Fourier transform ion cyclotron resonance mass spectra of natural organic matter. Analytical Chemistry, 78(13): 4363-4373. DOI:10.1021/ac0600306 |
Lawson EC, Bhatia MP, Wadham JL, et al. 2014. Continuous summer export of nitrogen-rich organic matter from the Greenland Ice sheet inferred by ultrahigh resolution mass spectrometry. Environmental Science & Technology, 48(24): 14248-14257. DOI:10.1021/es501732h |
Legrand M, Preunkert S, Wagenbach D, et al. 2003. A historical record of formate and acetate from a high-elevation Alpine glacier: Implications for their natural versus anthropogenic budgets at the European scale. Journal of Geophysical Research: Atmospheres, 108(D24): 4788. DOI:10.1029/2003JD003594 |
Legrand M, Preunkert S, Schock M, et al. 2007. Major 20th century changes of carbonaceous aerosol components (EC, WinOC, DOC, HULIS, carboxylic acids, and cellulose) derived from Alpine ice cores. Journal of Geophysical Research: Atmospheres, 112(D23): D23S11. DOI:10.1029/2006JD008080 |
Marsh JJS, Boschi VL, Sleighter RL, et al. 2013. Characterization of dissolved organic matter from a Greenland ice core by nanospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Journal of Glaciology, 59(214): 225-232. DOI:10.3189/2013JoG12J061 |
Mazzoleni LR, Ehrmann BM, Shen XH, et al. 2010. Water-soluble atmospheric organic matter in fog: exact masses and chemical formula identification by ultrahigh-resolution Fourier transform ion cyclotron resonance mass spectrometry. Environmental Science & Technology, 44(10): 3690-3697. DOI:10.1021/es903409k |
Miyazaki Y, Kawamura K, Jung J, et al. 2011. Latitudinal distributions of organic nitrogen and organic carbon in marine aerosols over the western North Pacific. Atmospheric Chemistry and Physics, 11(7): 3037-3049. DOI:10.5194/acp-11-3037-2011 |
Mopper K, Stubbins A, Ritchie JD, et al. 2007. Advanced instrumental approaches for characterization of marine dissolved organic matter: extraction techniques, mass spectrometry, and nuclear magnetic resonance spectroscopy. Chemical Reviews, 107(2): 419-442. DOI:10.1021/cr050359b |
Nebbioso A, Piccolo A. 2013. Molecular characterization of dissolved organic matter (DOM): a critical review. Analytical and Bioanalytical Chemistry, 405(1): 109-124. DOI:10.1007/s00216-012-6363-2 |
Ng NL, Kwan AJ, Surratt JD, et al. 2008. Secondary organic aerosol (SOA) formation from reaction of isoprene with nitrate radicals (NO3)
. Atmospheric Chemistry and Physics, 8(14): 4117-4140. DOI:10.5194/acp-8-4117-2008 |
Otto-Bliesner BL, Brady EC, Clauzet G, et al. 2006. Last glacial maximum and holocene climate in CCSM3. Journal of Climate, 19(11): 2526-2544. DOI:10.1175/JCLI3748.1 |
Singer GA, Fasching C, Wilhelm L, et al. 2012. Biogeochemically diverse organic matter in Alpine glaciers and its downstream fate. Nature Geoscience, 5(10): 710-714. DOI:10.1038/ngeo1581 |
Stubbins A, Spencer RGM, Chen HM, et al. 2010. Illuminated darkness: Molecular signatures of Congo River dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnology and Oceanography, 55(4): 1467-1477. DOI:10.4319/lo.2010.55.4.1467 |
Stubbins A, Hood E, Raymond PA, et al. 2012. Anthropogenic aerosols as a source of ancient dissolved organic matter in glaciers. Nature Geoscience, 5(3): 198-201. DOI:10.1038/ngeo1403 |
Surratt JD, Kroll JH, Kleindienst TE, et al. 2007. Evidence for organosulfates in secondary organic aerosol. Environmental Science & Technology, 41(2): 517-527. DOI:10.1021/es062081q |
Vairavamurthy A, Mopper K, 1989. Mechanistic Studies of organosulfur (Thiol) formation in coastal marine sediments. In: Saltzman ES, Cooper WJ (eds.). Biogenic Sulfur in the Environment. Washington DC: American Chemical Society, pp. 231–242.
|
Willoughby AS, Wozniak AS, Hatcher PG. 2014. A molecular-level approach for characterizing water-insoluble components of ambient organic aerosol particulates using ultrahigh-resolution mass spectrometry. Atmospheric Chemistry and Physics, 14(18): 10299-10314. DOI:10.5194/acp-14-10299-2014 |
Wolff EW, Barbante C, Becagli S, et al. 2010. Changes in environment over the last 800,000 years from chemical analysis of the EPICA Dome C ice core. Quaternary Science Reviews, 29(1–2): 285-295. DOI:10.1016/j.quascirev.2009.06.013 |
Wozniak AS, Willoughby AS, Gurganus SC, et al. 2014. Distinguishing molecular characteristics of aerosol water soluble organic matter from the 2011 trans-North Atlantic US GEOTRACES cruise. Atmospheric Chemistry and Physics, 14(16): 8419-8434. DOI:10.5194/acp-14-8419-2014 |
Xu JZ, Zhang Q, Wang ZB, et al. 2015. Chemical composition and size distribution of summertime PM2.5 at a high altitude remote location in the northeast of the Qinghai-Xizang (Tibet) Plateau: insights into aerosol sources and processing in free troposphere
. Atmospheric Chemistry and Physics, 15(9): 5069-5081. DOI:10.5194/acp-15-5069-2015 |
Yee LD, Kautzman KE, Loza CL, et al. 2013. Secondary organic aerosol formation from biomass burning intermediates: phenol and methoxyphenols. Atmospheric Chemistry and Physics, 13(16): 8019-8043. DOI:10.5194/acp-13-8019-2013 |
Zhao Y, Hallar AG, Mazzoleni LR. 2013. Atmospheric organic matter in clouds: exact masses and molecular formula identification using ultrahigh-resolution FT-ICR mass spectrometry. Atmospheric Chemistry and Physics, 13(24): 12343-12362. DOI:10.5194/acp-13-12343-2013 |
 2018, Vol. 10
2018, Vol. 10
