Article Information
- Fang Wang, HongLang Xiao, XiaoMei Peng, Shang Li . 2017.
- Effects of salt and alkali stress on Reaumuria soongorica germination
- Sciences in Cold and Arid Regions, 9(2): 158-166
- http://dx.doi.org/10.3724/SP.J.1226.2017.000158
Article History
- Received: October 1, 2016
- Accepted: January 17, 2017
2. Shaanxi University of Technology, Hanzhong, Shaanxi 723000, China
Soil salinization is a worldwide environmental problem. Inland saline soils generally contain both neutral and alkaline salts and often experience alkalization concurrently with salinization. In such habitats, plants are stressed not only by increasing salinity but also by alkalinity, with alkali stress having a more negative ecological effect than salt stress. Seed germination is the initial and most crucial stage in the life cycle of plants (Guan et al., 2009; Shi et al., 2016); successful plant establishment largely depends on successful germination (Tlig et al., 2008). The ability to maintain seed viability during exposure to saline and/or alkaline conditions, and to commence germination when such stresses are reduced or removed enables saline- and alkaline-tolerant species to persist under extreme abiotic conditions (Guan et al., 2009). Thus, seed structure and environmental conditions affect seed germination and the formation and succession of desert vegetation (Zhang et al., 2005; Wang et al., 2013). Tolerance to salinity during germination is critical for the establishment of plants growing in saline soils of arid regions (Khan and Gulzar, 2003). In the life cycle of a plant, the seed has the highest resistance to extreme environmental factors, whereas the seedling has the lowest environmental tolerance (Gutterman, 2002). Seeds that do not germinate in a stressed environment build up a seed bank in the soil, securing the long-term existence of the species by spreading germination over time and using opportunistically suitable germination conditions (Caballero et al., 2003).
Reaumuria soongorica, a dominant, constructive species of desert shrub vegetation in northwestern China, is widely distributed (Liu et al., 2007), tolerating many environmental conditions. It is drought resistant, salt tolerant, and important in dune fixation, all of which are important factors for maintaining the stability and continuity of the desert ecosystem (Ma et al., 2007).
Previous studies on R. soongorica focused mainly on how drought stress affects seed germination, while little attention has been paid to the effects of salt and alkali stress on germination. Thus, the main objectives of this study on R. soongorica were (1) to test the interactive effects of salt and alkali stress on seed germination and germination recovery, (2) to test the interactive effects of salt and alkali stress on early seedling growth, and (3) to analyze the features of salt–alkaline conditions.
2 Materials and methodsWe collected R. soongorica seeds from the vicinity of the Minqin Desert Botanical Garden in Gansu Province, Northwest China, in the autumn of 2010. We stored the seeds in envelopes. The botanical garden (103°51'E, 38°38'N, altitude 1,378 m) is located in the southeastern margin of Badain Jaran Desert. The area is a typical temperate continental desert climate with cold winters and hot summers that exhibit large diurnal temperature fluctuations. Annual average temperature is 7.6 °C, temperature extremes range from −30.8 °C to 40.0 °C, and there is a frost-free period of 175 days. Average annual precipitation is 113.2 mm, average annual evaporation is 2,604.3 mm, and average relative humidity is 47%. There is an average of 2,799.4 hours of sunshine per year and the active accumulated temperature greater than 10 °C is 3,036.4 °C. Winds are predominantly northwesterly in winter and there can be up to 83 days per year of blowing sand, mostly occurring from February to May. Average annual wind speed is 2.5 m/s and maximum wind speed is 23.0 m/s. The soil is alkaline sandy with a deep sand bed and poor fertility. Soil salinity is 0.146%, soil organic matter is 0.1975%, total nitrogen is 0.0079%, total phosphorus is 0.116%; and the pH is 8.3. Groundwater depth is below 15 m.
2.1 Treatment formulationsWe used two neutral salts (NaCl, Na 2SO 4) and two alkaline salts (NaHCO 3, Na 2CO 3) in our laboratory study (all reagents were analytical grade). We mixed the four salts in different proportions (Table 1) and divided them into six treatment groups (A, B, C, D, E, and F) based on ascending proportions of alkaline salt. Thus, we studied a total of 24 treatments with the salt content, pH from 6.53 to 11.14 and its variation similar to natural saline.
| Treatment | Salt composition and molar ratio | |||
| NaCl | Na 2SO 4 | NaHCO 3 | Na 2CO 3 | |
| A | 1 | 1 | 0 | 0 |
| B | 1 | 2 | 1 | 0 |
| C | 1 | 9 | 9 | 1 |
| D | 1 | 1 | 1 | 1 |
| E | 9 | 1 | 1 | 9 |
| F | 1 | 1 | 9 | 9 |
Table 2 lists the stress factor data for each treatment group. Based on R. soongorica's salinity tolerance, we set four salinity concentrations (50, 100, 200, and 300 mmol/L) for each group, respectively labeled 1, 2, 3, and 4. For example, A1 means that treatment for group A has a concentration of 50 mmol/L. Because the treatment solutions contain a variety of salts, they are buffered and show large pH changes between groups and smaller changes within groups. Thus, since pH is not sufficient to reflect the actual alkaline strength, we introduced a new stress index, buffer capacity. For our study, we defined buffer capacity as the mmol amount of H+ that was needed to reduce the pH of a liter of treatment solution to equal the control solution pH (Shi and Sheng, 2005). Of the six groups, the buffer capacity of groups A1–A4 was near zero, because these groups had no alkaline salts. The buffering capacity of the remaining groups increases with increasing proportions of alkaline salts and salt concentrations. If considered from the concentration of ion Na+ that mainly cause damage, concentration of Na+ in four concentrations in each group is followed by 75.0, 150.0, 300.0, 450.0 mmol/L.
| Treatment | Salinity (mmol) | pH | Buffer capacity (mmol) | [Na+] (mmol) | [Cl−](mmol) | [SO 42−] (mmol) | [HCO 3−] (mmol) | [CO 32−] (mmol) | 2[CO 32−]+[HCO 3−] (mmol) |
| A1 | 50 | 6.53 | 0.01 | 75.0 | 25.0 | 25.0 | 0.0 | 0.0 | 0.0 |
| B1 | 50 | 7.51 | 7.50 | 75.0 | 12.5 | 25.0 | 12.5 | 0.0 | 12.5 |
| C1 | 50 | 8.69 | 19.50 | 75.0 | 2.5 | 22.5 | 22.5 | 2.5 | 27.5 |
| D1 | 50 | 9.65 | 30.50 | 75.0 | 12.5 | 12.5 | 12.5 | 12.5 | 37.5 |
| E1 | 50 | 10.59 | 43.00 | 75.0 | 22.5 | 2.5 | 2.5 | 22.5 | 47.5 |
| F1 | 50 | 10.92 | 52.00 | 75.0 | 2.5 | 2.5 | 22.5 | 22.5 | 67.5 |
| A2 | 100 | 6.60 | 0.02 | 150.0 | 50.0 | 50.0 | 0.0 | 0.0 | 0.0 |
| B2 | 100 | 7.66 | 17.50 | 150.0 | 25.0 | 50.0 | 25.0 | 0.0 | 25.0 |
| C2 | 100 | 8.76 | 39.00 | 150.0 | 5.0 | 45.0 | 45.0 | 5.0 | 55.0 |
| D2 | 100 | 9.78 | 55.50 | 150.0 | 25.0 | 25.0 | 25.0 | 25.0 | 75.0 |
| E2 | 100 | 10.63 | 81.50 | 150.0 | 45.0 | 5.0 | 5.0 | 45.0 | 95.0 |
| F2 | 100 | 10.96 | 109.50 | 150.0 | 5.0 | 5.0 | 45.0 | 45.0 | 135.0 |
| A3 | 200 | 6.64 | 0.04 | 300.0 | 100.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| B3 | 200 | 7.79 | 33.00 | 300.0 | 50.0 | 100.0 | 50.0 | 0.0 | 50.0 |
| C3 | 200 | 8.83 | 82.00 | 300.0 | 10.0 | 90.0 | 90.0 | 10.0 | 110.0 |
| D3 | 200 | 9.88 | 113.00 | 300.0 | 50.0 | 50.0 | 50.0 | 50.0 | 150.0 |
| E3 | 200 | 10.66 | 167.50 | 300.0 | 90.0 | 10.0 | 10.0 | 90.0 | 190.0 |
| F3 | 200 | 11.03 | 221.00 | 300.0 | 10.0 | 10.0 | 90.0 | 90.0 | 270.0 |
| A4 | 300 | 6.75 | 0.06 | 450.0 | 150.0 | 150.0 | 0.0 | 0.0 | 0.0 |
| B4 | 300 | 7.97 | 52.00 | 450.0 | 75.0 | 150.0 | 75.0 | 0.0 | 75.0 |
| C4 | 300 | 8.88 | 129.00 | 450.0 | 15.0 | 135.0 | 135.0 | 15.0 | 165.0 |
| D4 | 300 | 9.94 | 164.00 | 450.0 | 75.0 | 75.0 | 75.0 | 75.0 | 225.0 |
| E4 | 300 | 10.72 | 243.50 | 450.0 | 135.0 | 15.0 | 15.0 | 135.0 | 285.0 |
| F4 | 300 | 11.14 | 260.00 | 450.0 | 15.0 | 15.0 | 135.0 | 135.0 | 405.0 |
We used the Petri dish-paper germination method for seed germination at room temperature (23±2 °C). We chose plump, clean seeds soaked in clean water for 10 hours, rinsing with distilled water after being sterilized by 3% H 2O 2 for 15~20 min, and then draining moisture. Placed two layers of filter paper in clean and dry petri dishes (90 mm diameter), respectively adding the treatment solution to saturated filter paper (with an equal amount of distilled water as a control treatment). Processed Reaumuria soongorica seeds where evenly placed in petri dishes. Distilled water was added every day to maintain a constant concentration of the salt solution by gravimetric method. The seed experiments were replicated three times with 25 seeds per treatment. We performed a statistic daily germination count, and observe continuous observation for 10 days. Later, healthy seeds that did not germinate were transferred to distilled water after washing out salinity with distilled water, then restored germination under the same conditions for 7 d; recorded number of germinating seeds. Germination was defined when radicle broke through testa and exceeded half the length of the seed.
2.4 Measurement indicatorsUsing Khan and Ungar's (1997) methodology, we calculated the germination index, GI, with the following formulae:
Initial germination rate (%) = (b/c) × 100
Recovery germination rate (%) = [(a−b)/(c−b)] × 100
Final germination rate (%) = (a/c) × 100
where, a is the total number of germinated seeds, b is the number of seeds germinated in salt solution, and c is the number of all seeds in the particular treatment. The germination index is defined as
GI = ∑n i /D i
where, n i is the total number of germinated seeds on the ith day and D i is the number of corresponding days of germination.
On the seventh day of germination, we randomly selected five seedlings from each treatment (for treatments with less than five seedlings, we took all the seedlings) and measured the length of the primary root and germ (including hypocotyl and terminal bud).
2.5 Factor analysisWe calculated the salinity of each treatment solution and the concentration of hydronium, such as Na+, Cl−, SO 42−, HCO 3−, and CO 32−, based on the actual concentration and the proportion of treatment solution. We measured the pH of each treatment solution with a digital pH meter. Using hydrochloric acid titration to measure the buffer capacity of each treatment solution, we titrated 100 mL of treatment solution with 1 mol/L HCI and used a pH meter to monitor the changes in pH. We used the pH of the control solution as the titration end point in order to determine the buffer capacity of each treatment solution.
2.6 Statistical analysisWe completed data processing and statistical analyses using SPSS v. 16.0 software (SPSS Inc., Chicago, IL, USA). The effect of different salt stress treatments on germination index of R. soongorica seeds was tested by Analysis of Variance (ANOVA). In case of a significant effect difference, further multiple comparisons were performed. Data is represented by the average of three replicates and its standard error (SE), and inspection level is 5%.
3 Results and analysis 3.1 Effects of mixed salt stresses on initial germinatiion rateFigure 1 shows changes of initial germination rate over time under different mixed salt stress and illustrates that salinity has a negative effect on the initial germination rate in all groups. As the salt concentration increases, the initial germination rate of R. soongorica seeds exhibit an overall downward trend, and the time to reach the maximum germination rate is also delayed. Germination curve of each concentration in the first four days increases, and then levels out, almost parallels with the horizontal axis. When the salt concentration is 50 mmol/L, the germination rate of the treatment group is higher than the control group in groups B, C, and D, although all treatment and control groups are not significantly different at this concentration (Table 3). When the concentration reaches 200 mmol/L, the germination rate of R. soongorica seeds is severely inhibited, with all groups lower than 20% (except group B). At concentrations of 300 mmol/L, the germination rate is near zero for all but group B.
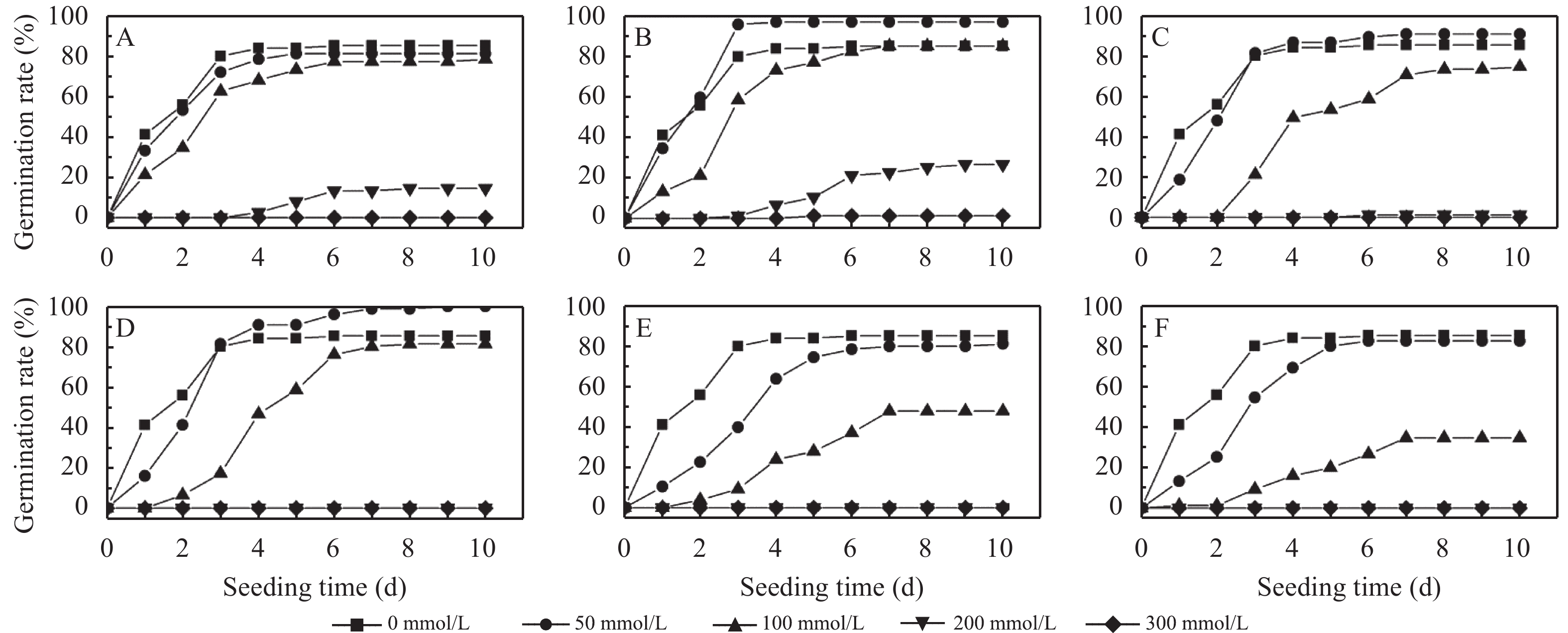
|
| Figure 1 Changes to germination rate of Reaumuria soongorica seeds under mixed salt stress over time (Treatments A–F) |
| Treatment | Salinity (mmol/L) | Germinating rate (%) | ||
| Initial germination rate | Recovery germination rate | Final germination rate | ||
| A | 0 | 85.3±9.3a | 0±0b | 85.3±9.3a |
| 50 | 81.3±8.7a | 0±0b | 81.3±8.7a | |
| 100 | 78.7±7.4a | 3.7±3.7b | 80.0±6.1a | |
| 200 | 14.7±2.7b | 81.1±5.0a | 84.0±4.0a | |
| 300 | 0±0b | 81.3±2.7a | 81.3±2.7a | |
| B | 0 | 85.3±9.3a | 0±0b | 85.3±9.3abc |
| 50 | 97.3±2.7a | 16.7±16.7b | 98.7±1.3a | |
| 100 | 85.3±1.3a | 58.3±22a | 93.3±3.5ab | |
| 200 | 26.7±3.5b | 69.4±3.6a | 77.3±3.5bc | |
| 300 | 1.3±1.3c | 72.9±5.6a | 73.3±5.3c | |
| C | 0 | 85.3±9.3a | 0±0b | 85.3±9.3ab |
| 50 | 90.7±3.5a | 33.3±33.3ab | 92.0±4.6a | |
| 100 | 74.7±9.3a | 39.5±6.2ab | 84.0±6.1ab | |
| 200 | 1.3±1.3c | 55.3±4.4a | 56.0±4.0b | |
| 300 | 0±0d | 69.3±3.5a | 69.3±3.5b | |
| D | 0 | 85.3±9.3ab | 0±0d | 85.3±9.3ab |
| 50 | 100.0±0.0a | 0±0d | 100.0±0.0a | |
| 100 | 81.3±5.8b | 28.1±11.1c | 85.3±5.8ab | |
| 200 | 0±0c | 48.0±6.1b | 48.0±6.1c | |
| 300 | 0±0c | 74.7±3.5a | 74.7±3.5b | |
| E | 0 | 85.3±9.3a | 0±0c | 85.3±9.3a |
| 50 | 81.3±6.7a | 0±0c | 81.3±6.7ab | |
| 100 | 48.0±6.1b | 32.3±8.1b | 65.3±3.5b | |
| 200 | 0±0c | 64.0±4.6a | 64.0±4.6b | |
| 300 | 0±0c | 73.3±5.8a | 73.3±5.8ab | |
| F | 0 | 85.3±9.3a | 0±0c | 85.3±9.3a |
| 50 | 82.7±7.4a | 0±0c | 82.7±7.4a | |
| 100 | 34.7±10.9b | 13.8±4.2b | 44.0±9.2b | |
| 200 | 0±0c | 70.7±1.3a | 70.7±1.3a | |
| 300 | 0±0c | 65.3±3.5a | 65.3±3.5ab | |
| Note: Carry on ANOVA for each column of data in the table respectively, P < 0.05, the average marked the same letter in the same column has no significant differences in each processing | ||||
Seeds deteriorate before losing germinating power and this is reflected in the GI. Thus, the GI is more sensitive to seed vigor than the germination rate. Our results show that changes to the GI and initial germination rate of R. soongorica seeds have similar trends (Figure 2). As the salt concentration increases, the GI of R. soongorica seeds exhibit a decreasing trend (except group B at 50 mmol/L which has a GI higher than the control group). From group A to E, with proportion of alkaline salts increasing, pH value and the decline of germinating index increases.
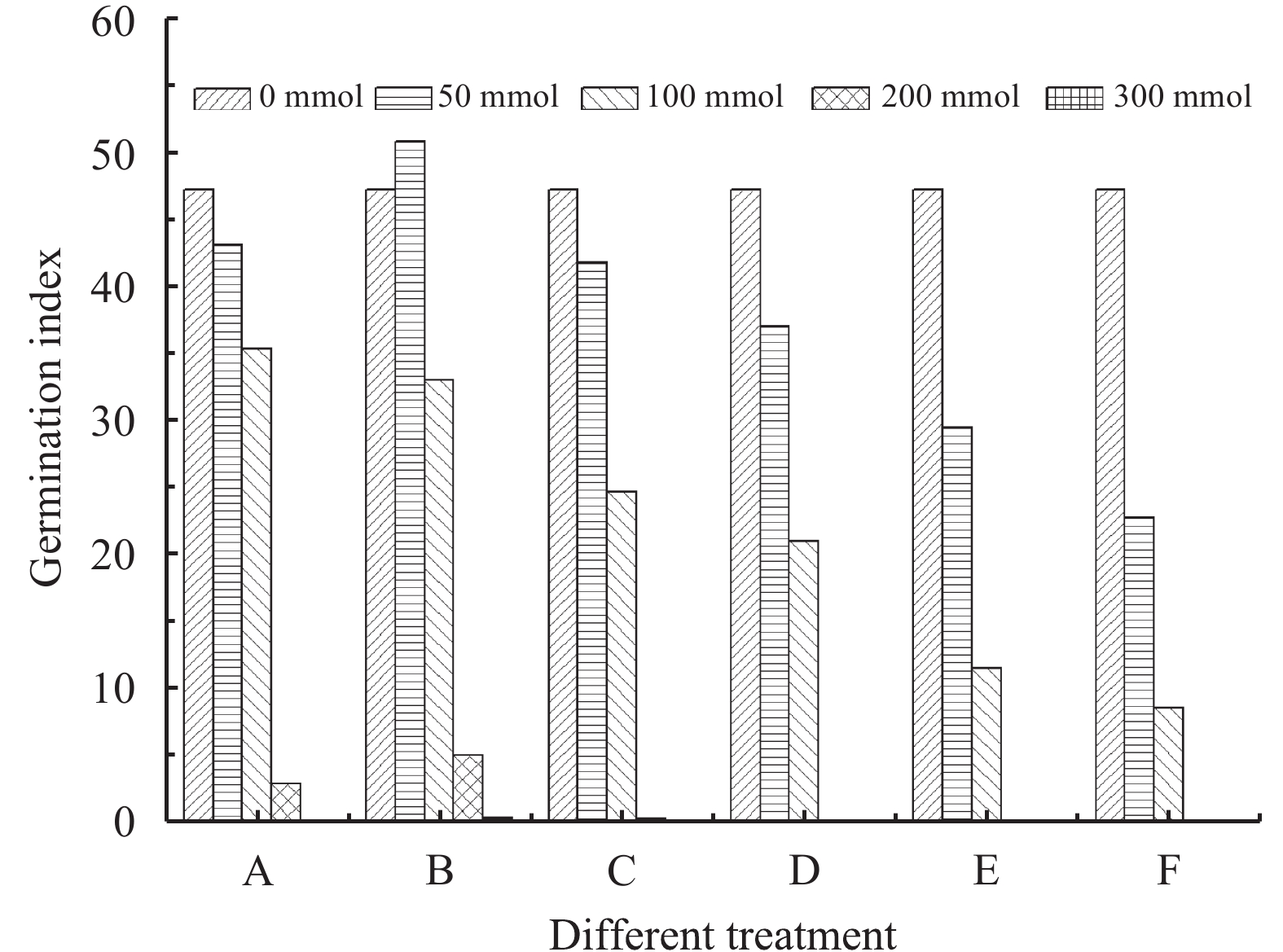
|
| Figure 2 Effects of salt concentration on germination index of Reaumuria soongorica seeds under differing mixed salt stress |
Ten days after the stress treatments, we shifted any ungerminated, healthy seeds to distilled water. The recovery germination rate of R. soongorica seeds is presented in Table 3. As the salt concentration increases, the recovery germination rate shows an increasing trend, but the final germination rate shows a downward trend. For all groups, as the proportion of alkaline salts increases, the pH increases, but the final germination rate still shows a downward trend and the recovery germination rate has no significant regularity.
3.4 Effects of mixed salt stress on Reaumuria soongorica embryoMixed salt stress significantly affected the growth of R. soongorica germ and radicle, with a more significant effect on the radicle (Table 4, Figures 3 and 4). Further analyses indicate that as the salt concentration increases, the radicle length of R. soongorica significantly decreases. From group A to E, as the proportion of alkali salt and pH increases, the decrease in radicle length became more apparent; as the salt concentration increased, the extent of the decrease became more rapid. The germ length also decreased with increased salt concentrations. In groups A and B, the germ length at a 50 mmol/L salt concentration is higher than that germinated in the control solution, and in group A the germ length at a 100 mmol/L salt concentration is higher than the control solution germ. This indicates that a certain amount of salt stress and increased pH can accelerate the growth of germs.
| Treatment | Salinity (mmol) | Length of germ (cm) | Length of radicle (cm) |
| A | 0 | 1.18±0.11c | 3.63±0.52a |
| 50 | 1.78±0.21a | 3.54±0.45a | |
| 100 | 1.45±0.30b | 2.72±0.48b | |
| 200 | 0.79±0.25d | 0.65±0.83c | |
| 300 | 0±0e | 0±0d | |
| B | 0 | 1.18±0.11b | 3.63±0.52a |
| 50 | 1.77±0.15a | 1.99±0.62b | |
| 100 | 1.06±0.13b | 0.87±0.02c | |
| 200 | 0.42±0.06c | 0.06±0.03c | |
| 300 | 0.19±0.00c | 0±0c | |
| C | 0 | 1.18±0.11a | 3.63±0.52a |
| 50 | 1.03±0.15b | 1.10±0.10b | |
| 100 | 0.41±0.01c | 0.05±0.05c | |
| 200 | 0.17±0.00d | 0±0c | |
| 300 | 0±0d | 0±0c | |
| D | 0 | 1.18±0.11a | 3.63±0.52a |
| 50 | 0.78±0.17b | 0.64±0.16b | |
| 100 | 0.43±0.08c | 0.07±0.06c | |
| 200 | 0±0d | 0±0c | |
| 300 | 0±0d | 0±0c | |
| E | 0 | 1.18±0.11a | 3.63±0.52a |
| 50 | 0.76±0.1b | 0.58±0.21b | |
| 100 | 0.38±0.02c | 0±0c | |
| 200 | 0±0d | 0±0c | |
| 300 | 0±0d | 0±0c | |
| F | 0 | 1.18±0.11a | 3.63±0.52a |
| 50 | 0.66±0.10b | 0.24±0.03b | |
| 100 | 0.42±0.12c | 0±0b | |
| 200 | 0±0d | 0±0b | |
| 300 | 0±0d | 0±0b | |
| Note: Carry on ANOVA for each column of data in the table respectively, P < 0.05, the average marked the same letter in the same column has no significant differences in each processing | |||
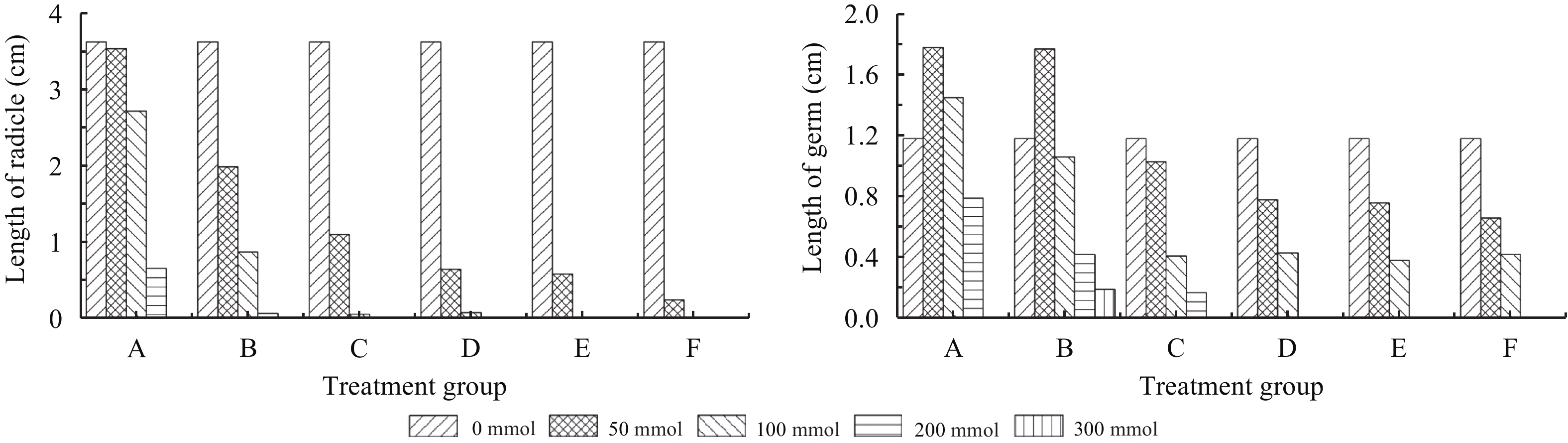
|
| Figure 3 Effects of salinity and alkalinity on length of germ and radicle of Reaumuria soongorica seeds under mixed salt stress |

|
| Figure 4 Contrast in germ growth of Reaumuria soongorica under mixed salt stress |
The main stress factors of each treatment are presented in Table 2. To further analyze quantitative relationships, we performed a stepwise multiple regression analysis between stress indices (germination rate, GI, length of germ, length of radicle) and main stress factors. Results of the regression analyses show that all indices have significant correlation to multivariate linear equations (Table 5). For the germination rate of Reaumuria soongorica seeds, salt concentration as the only dominant factor is retained, that is the decisive dominant factor in inhibiting germination of Reaumuria soongorica seeds, and other factors are not significant. However, for two indices of embryo growth (length of germ and radicle), the three main stress factors (salinity, buffer capacity and pH) are retained. Thus, in the process of embryo growth, three stress factors have different degrees of effect, where salinity has the largest importance.
| Index | Model | R2 | Variance test | β 1 | β 2 | β 3 |
| Germination rate | Y=86.521−0.0.381X 1 | 0.80 | P<0.001 | −0.90 | ||
| Germination index | Y=62.215−0.154X 1−2.699X 3 | 0.79 | P<0.001 | −0.82 | −0.24 | |
| Length of germ | Y=3.293−0.005X 1+0.002X 2−0.237X 3 | 0.77 | P<0.001 | −0.71 | 0.21 | −0.70 |
| Length of radicle | Y=9.504−0.013X 1+0.016X 2−0.896X 3 | 0.86 | P<0.001 | −0.88 | 0.78 | −1.12 |
| Arguments: X 1=salinity; X 2=buffer capacity; X 3=pH. β 1–β 3: Standardized regression coefficients corresponding to each variable (X 1–X 3) The larger the standard regression coefficient, the stronger the argument importance. R2=correlation coefficient | ||||||
Our study shows that the germination rate of R. soongorica seeds exhibits a downward trend with increasing salt concentration, although a certain amount of salt and increased pH resulted in initial germination rates higher than those of the control group, indicating that low salt stress promotes R. soongorica germination. Almost all ungerminated seeds transferred to distilled water after 10 days of salt stress exhibited restored germination. Similar to the findings of El-Keblawy (2004), Garcia-Tiburcio and Troyo-Dieguez (1993), Huang et al. (2003), and Redondo et al. (2004), our results indicate that salt stress can cause the seeds of halophytes to become temporarily dormant, but its germination capacity has not been completely lost.
The degree of contact between soil and root is the most important factor influencing the transmission of moisture. Under our experimental conditions, seeds germinated without soil contact. Moreover, for some species, seed germination rate of 85% or higher is attributed to moisture adsorbed directly from water vapor (Wuest, 2002, 2007). When humidity is restricted or salinization increases, dormancy reduces the risk of excessive seed deaths, especially in conditions of moderate salinity (Redondo et al., 2004; Gorai and Neffati, 2007).
Further analysis shows that the effects of mixed salt stress on the germination rate of R. soongorica seeds are consistent with those for a single salt: the germination rate decreases as the salt concentration increases. However, the stress factors with mixed salt concentrations are much more complicated than with a single salt. For germination rates, statistical analyses show that salinity is the dominant factor, while pH and buffer capacity which reflect alkali stress intensity have little effect. Thus, salt stress is a major contra-indication during the seed germination stage, but alkali stress has little influence on seed germination.
Salt stress affects plants through ion toxicity and osmotic stress. The complex mixtures of salt in this study not only involve ion toxicity and osmotic stress, but also pH stress. The pH stress can damage the mineral nutrition around the seeds and directly affect the absorption of mineral elements. However, during the seed germination stage, the main exchange with the physical environment is moisture absorption; because this stage seldom includes the absorption of mineral elements from the environment, pH has little effect. Osmotic stress is the main factor at the seed germination stage. Moisture absorption is key for germination and salt concentration determines absorption, thus, whether a single salt or a mixed salt, salt concentration is the dominant factor.
During the seedling stage, however, mixed salt concentrations will affect radicle growth, germ growth, cell division, elongation, and differentiation. The seedling must obtain minerals from the environment, and plants not only need osmoregulation, but also pH adjustment. The role of buffer capacity, pH, and other factors may gradually increase, and even become dominant. Studies have demonstrated that buffer capacity and salt concentration are dominant factors affecting the growth of sunflowers and L. chinensis seedlings (Shi and Sheng, 2005; Shi and Wang, 2005), with buffer capacity having a larger impact than salinity. Our study confirmed that, under mixed salt stress, pH and buffering capacity, in addition to salinity, have significant effects on the growth of the R. soongorica radicle and germ. Under salinity stress, the growth stages and metabolic characteristics differ, and the dominant factor causing environmental stress will differ.
Our study shows that R. soongorica seeds under high salinity conditions exhibit not only low germination rates, but also delayed germination. After being subjected to increased salt stress, almost all ungerminated R. soongorica seeds exhibited restored germination, suggesting that seeds will not be damaged in a highly saline soil environment, but will delay germination until adequate moisture conditions occur. This is likely a mechanism thatR. soongorica seeds use to resist salinity and avoid mass seed death. At low salinities, the germinating rate is high and the GI is relatively high; seeds germinate quickly. The low saline environment is relatively suitable and seeds germinate quickly in order to relieve the negative effects of environmental change. This may be a mechanism for seeds to adapt to the changing environment. Compared with the germination capacity, seed viability under high salinity is a better standard to measure salt tolerance. If the seeds lose viability at high salinity, they cannot adapt to saline habitats. Halophyte seeds remain viable in high salt environments and have the ability to continue germination when the stress reduces. That ability determines which plants are successful in saline environments.
Acknowledgments:This work was supported by the National Natural Science Foundation of China (41401043, 91125025).
| Caballero I, Olano JM, Loidi J, et al, 2003. Seed bank structure along a semi-arid gypsum gradient in Central Spain. Journal of Arid Environments, 55: 287–299. DOI: 10.1016/S0140-1963(03)00029-6 |
| El-Keblawy A, 2004. Salinity effects on seed germination of the common desert range grass, Panicum turgidum. Seed Science and Technology, 32: 873–878. DOI: 10.15258/sst |
| Garcia-Tiburcio H, Troyo-Dieguez E, 1993. Effects of sudden drop in salinity on the germination of Salicornia bigelowii Torr in the laboratory. Phyton-International Journal of Experimental Botany, 54: 127–137. |
| Gorai M, Neffati M, 2007. Germination responses of Reaumuria vermiculata to salinity and temperature. Annals of Applied Biology, 151: 53–59. DOI: 10.1111/aab.2007.151.issue-1 |
| Guan B, Zhou D, Zhang H, et al, 2009. Germination responses of Medicago ruthenica seeds to salinity, alkalinity, and temperature. Journal of Arid Environments, 73: 135–138. DOI: 10.1016/j.jaridenv.2008.08.009 |
| Gutterman Y, 2002. Survival Strategies of Annual Desert Plants: Adaptations of Desert Organisms, Preface. Springer, Berlin. |
| Huang ZY, Zhang XS, Zheng GH, et al, 2003. Influence of light, temperature, salinity and storage on seed germination of Haloxylon ammodendron. Journal of Arid Environments, 55: 453–464. DOI: 10.1016/S0140-1963(02)00294-X |
| Khan MA, Gulzar S, 2003. Germination responses of Sporobolus ioclados: a saline desert grass. Journal of Arid Environments, 53: 387–394. DOI: 10.1006/jare.2002.1045 |
| Khan MA, Ungar IA, 1997. Effects of thermoperiod on recovery of seed germination of halophytes from saline conditions. American Journal of Botany, 84: 279–283. DOI: 10.2307/2446089 |
| Liu YB, Zhang TG, Li XR, et al, 2007. Protective mechanism of desiccation tolerance in Reaumuria soongorica: Leaf abscission and sucrose accumulation in the stem. Science in China (Series C: Life Sciences), 50: 15–21. DOI: 10.1007/s11427-007-0002-8 |
| Ma JY, Chen K, Xia DS, et al, 2007. Variation in foliar stable carbon isotope among populations of a desert plant, Reaumuria soongorica (Pall.) Maxim. in different environments. Journal of Arid Environments, 69: 365–374. DOI: 10.1016/j.jaridenv.2006.11.002 |
| Redondo S, Rubio-Casal AE, Castillo JM, et al, 2004. Influences of salinity and light on germination of three Sarcocornia taxa with contrasted habitats. Aquatic Botany, 78: 255–264. DOI: 10.1016/j.aquabot.2003.11.002 |
| Shi D, Sheng Y, 2005. Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environmental and Experimental Botany, 54: 8–21. DOI: 10.1016/j.envexpbot.2004.05.003 |
| Shi D, Wang D, 2005. Effects of various salt-alkali mixed stresses on Aneurolepidium chinense (Trin.) Kitag. Plant and Soil, 271: 15–26. DOI: 10.1007/s11104-004-1307-z |
| Shi Y, Liu Y, Yin HX, et al, 2016. Seed germination characteristics and local adaptation of resumuria soongarica. Journal of Desert Research, 36(3): 644–650. |
| Tlig T, Gorai M, Neffati M, 2008. Germination responses of Diplotaxis harra to temperature and salinity. Flora-Morphology, Distribution, Functional Ecology of Plants, 203: 421–428. DOI: 10.1016/j.flora.2007.07.002 |
| Wang Y, Jiang GQ, Han YN, et al, 2013. Effects of salt, alkali and salt-alkali mixed stresses on seed germination of the halophyte Salsola ferganica (Chenopodiaceae). Acta Ecologica Sinica, 33: 354–360. DOI: 10.1016/j.chnaes.2013.09.010 |
| Wuest SB, 2002. Water transfer from soil to seed: the role of vapor transport. Soil Science Society of America Journal, 66: 1760–1763. DOI: 10.2136/sssaj2002.1760 |
| Wuest S, 2007. Vapour is the principal source of water imbibed by seeds in unsaturated soils. Seed Science Research, 17: 3–9. DOI: 10.1017/S0960258507383165 |
| Zhang Y, Xue LG, Gao TP, et al, 2005. Research advance on seed germination of desert plants. Journal of Desert Research, 25: 106–112. |
 2017, 9
2017, 9


