Article Information
- XiaoMei Peng, ShengChun Xiao, GuoDong Cheng, QuanYan Tian, Xiao HongLang . 2017.
- Microcoring and dendrometer-detected intra-annual wood formation of Populus euphratica in the Ejina Oasis, northwestern China
- Sciences in Cold and Arid Regions, 9(1): 54-66
- http://dx.doi.org/10.3724/SP.J.1226.2017.00054
Article History
- Received: August 19, 2016
- Accepted: October 17, 2016
2. University of the Chinese Academy of Sciences, Beijing 100049, China
Seasonal stem radial growth and annual tree ring formation in trees has been studied for a long time (Fritts, 1976), but this field has taken on new energy due to its applications in dendrochronology (Speer, 2010). Recent studies of wood formation on a cellular level in a range of tree species (mainly conifers) have confirmed the significance of this knowledge for dendroclimatological and dendroecological studies (Deslauriers et al., 2003a, 2008; Gričar et al., 2006; Rossi et al., 2006a, 2007, 2008; Ren et al., 2015). However, these studies have concentrated on coniferous tree species in high-altitude or high-latitude regions. For arid-zone riparian forest, there is little detailed information on the intra-annual variation of wood formation and its correlation with environmental factors (Xiao et al., 2014a).
Riparian forest is usually a key component of riparian zones. The riparian zone forms an interface between terrestrial and aquatic ecosystems, and is also important because of its uniquely productive, physically dynamic, and biologically diverse plant communities (Ward et al., 2002; Stella et al., 2013). Over the last several decades, riparian forests have become increasingly vulnerable to the increasing pressures created around the world by human activities (e.g., changes in land use, watershed management to regulate runoff) and by climate change (Allan et al., 1997; Nilsson and Berggren, 2000; Zhang et al., 2012). In addition to their importance in forestry and in the local ecosystem, riparian forests are interesting sites for dendrochronological research (Stromberg, 2001; Edmondson et al., 2014).
Populus species are one of the most common trees in riparian ecosystems throughout the world's arid and semiarid regions, and have been used in many dendrochronological studies (Scott et al., 1999; Lite and Stromberg, 2005; Singer et al., 2013; Stella et al., 2013). In northwestern China, Populus euphratica is one of the major tree species in semi-arid to hyper-arid riparian forests, and has therefore been the subject of dendrochronological studies. Both the river runoff and groundwater depth were significantly correlated with long-term annual-resolution tree ring width series (Sun et al., 2006; Liu et al., 2007a; Peng et al., 2015) or with intra-annual variations in stem radial growth (Xiao et al., 2014a). In this arid region of water shortage, based on studies of water resources utilization of desert riparian forests, some studies were conducted to explore the reasonable spatial allocation of water recourses (Ye et al., 2007; Si et al., 2013; Bai et al., 2015). However, few studies focused on temporal allocation of water recourses. We therefore hypothesized that the intra-annual study of P. euphratica could reveal its relationships with environment factors, especially the hydrological ones, in a new way.
The variations in intra-annual tree ring formation can be tracked directly by using the microcoring technique (Rossi et al., 2006b), which is based on extracting xylem samples at short intervals. Using these samples, the state of tree ring formation at the time of the microcoring can be determined microscopically (Deslauriers et al., 2003b; González-González et al., 2013). Alternatively, point and band dendrometers have traditionally been used to monitor the dynamics of a stem's radius (Fritts, 1976; Drew and Downes, 2009). Stem radial increments (SRI) derived from dendrometer measurements have been widely used as representative estimates of the ring-width increase (RWI) (Deslauriers et al., 2011; Wang et al., 2015). However, dendrometer measurements do not distinguish between xylem, phloem, and periderm increments and are confounded by swelling and shrinkage of the overall stem diameter in response to changes in stem water content or temperature (Downes et al., 1999; Zweifel et al., 2000, 2001; Sevanto et al., 2011). On the other hand, the microcoring method is much more laborious than dendrometer measurements, which severely restricts its applicability for collecting large datasets. Given the advantages and disadvantages of dendrometers and microcoring, Maekinen (2008) suggested that the two methods be used in combination.
In an effort to profit from the advantages of dendrometers and microcoring and compensate for their disadvantages, we combined the two approaches to study the intra-annual wood formation of riparian P. euphratica. This study was carried out in a typical riparian forest site in northwestern China's Ejina Oasis, an extremely arid desert area. Our objectives are to describe the seasonal dynamics of cambial activity and tree ring formation on a cellular level (including the timing of growth initiation, maximum growth, and growth cessation), and to compare the seasonal dynamics of tree ring formation with the simultaneous variations in key environmental factors to detect the possible causes of these growth variations. A comparison between the methods of microcoring and point dendrometers was also an objective, given the usefulness and reliability of the two methods is still a hot debate.
2 Materials and methods 2.1 Study areaOur study was carried out in a natural riparian forest in the Ejina Oasis of northwestern China (97°10'23"E to 103°07'15"E, 39°52'20"N to 42°47'20"N) in the lower reaches of the Heihe River (Figure 1). The study area has a hyper-arid continental climate. The mean annual precipitation (1957 to 2014, at the national weather station at Ejina Banner, Inner Mongolia) is only 37.2 mm, but pan evaporation is 3, 706.3 mm. The mean annual air temperature is 8.8 °C, with the warmest month (July) having an average temperature of 27.0 °C, and the coldest month (January) having an average temperature of -11.6 °C. The zonal soil type is a gray-brown desert soil, with a forest-shrub meadow soil that alternates with fixed and semi-fixed sandy soil in the riparian forest (Xiao et al., 2014a). The Ejina Oasis is a small area that surrounds the main stream and the tributaries and distributaries of the Heihe River. Outside the Ejina Oasis, a gobi (gravel surface) peneplain and desert steppe are the primary landscape features. In the riparian forest, the dominant tree species is P. euphratica and the primary shrub species is Tamarix ramosissima Ledeb. (Cheng et al., 2009). The poplar trees form pure forest stands along the banks of the Heihe River and its tributaries and distributaries. The runoff from the river's middle reaches is the major source of surface water for the lower reaches, and plays a vital role in maintenance of groundwater levels in the riparian forest (Xi et al., 2010; Wang et al., 2013).
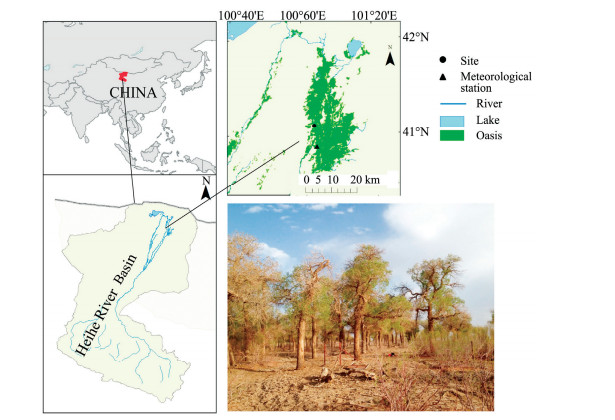
|
| Figure 1 Location of the sampling site and nearby Ejina meteorological station in the Ejina Oasis (an extremely arid area of northwestern China), and photograph of the study site in the lower reaches of the Heihe River Basin |
Sample trees without visible damage were selected from the dominant tree layer at the study site in the Ejina Oasis (Figure 1). Four poplar trees (D1, D2, D3, and D4) were monitored for variations in the stem radius from April 21 to October 15, 2013 using dendrometers. We installed DR Point dendrometers (Ecomatik, Munich, Germany) at a stem height of about 1.3 to 1.5 m above the ground. To ensure a smooth contact between the dendrometer's band and the trunk, the outer bark was slightly brushed off. The output of the dendrometers was stored at 1-h intervals using a datalogger. The later parts of the data for trees D1 and D3 was unusable because of a dendrometer error. During the same period, we obtained microcores (2.5 mm in diameter and 20 to 25 mm in length) from the stems of three other poplar trees (M1, M2, and M3) at a height of 1.0 to 1.3 m using a Trephor tool (Rossi et al., 2006b) at 1-week intervals from April 21 to September 15, 2013, and at 2-week intervals thereafter (i.e., on September 30 and October 15). All trees were located within a 10m×10m area, at a similar elevation above the river (Figure 1); thus, climatic conditions and groundwater levels should have been comparable for all trees.
The average tree diameter was about 50 cm for the four trees monitored using dendrometers, and was about 40 cm for the microcoring trees. The average tree height was about 10 m, and the trees averaged about 100 years old (based on core samples of other trees in the same stand). Groundwater levels were recorded at 1-h intervals that corresponded to the dendrometer measurement frequency using two 3001 LT Levelogger Gold monitors (Solinst, Georgetown, ON, Canada) installed in a well located within 10 m and with a similar elevation from the sampled trees.
The microcores were fixed in formalin-acetic acid-ethanol solution (at a ratio of 90:5:5 v/v) to prevent tissue deterioration. In the laboratory, samples were dehydrated with a graded series of ethanol and xylene, and were then embedded in paraffin (Zhang et al., 2013; Xiao et al., 2014b). Transverse sections 10 to 16 μm in thickness were cut from the samples with a rotary microtome. The sections were double-stained with 1% safranine (in purified water) and 0.5% Astra blue (in 95% ethanol), and permanently fixed to glass slides. All slides were examined with visible light under a microscope (Olympus CX31, Olympus America Inc., Center Valley, PA, USA), then the slide was photographed to enable analysis of wood formation processes (Figure 2).
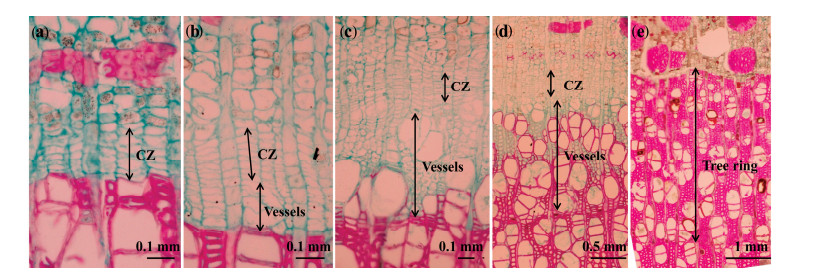
|
| Figure 2 Phases of wood formation in P. euphraticain 2013. (a) the reactive cambial zone (CZ) on April 21, with no new xylem cell production observed; (b) the initiation of vessel growth on May 12 and about the same length of cambial zone to that in (a); (c) the beginning of wall-thickening of the vessels on May 26, with the vessels occupying about four times the space of the cambial zone; (d) the enlargement and maturation of vessels on June 16; and (e) the current tree ring consists entirely of mature vessels on August 27 |
For each tree that was examined using the microcoring method, we considered three phases in our assessment of the phenology of tree ring formation: initiation and cessation of tree ring formation, and the end of vessel division. In spring, we defined the start of tree ring formation as the date when at least one radial file of enlarging vessels was observed. When no new vessels were produced, we considered vessel division to be finished. Tree ring formation was considered to be complete when the last cells had matured, with the cells stained completely red (Figure 2e) at the end of the growing period. For each section, the numbers and widths of the vessels and cambial cells were counted or calculated along five radial directions using the Image J software (National Institutes of Health, Bethesda, Maryland, USA; http://rsb.info.nih.gov/ij/index.html). The weekly RWI was defined as the average widths of the region containing xylem elements at a given point of time in the current year's growth ring.
We defined the daily cycle of stem radius changes and the daily SRI (Downes et al., 1999; Deslauriers et al., 2003a) using a three-step procedure that used two routines in the SAS software (SAS Institute, Inc., Cary, NC, USA) that had been developed to analyze hourly automatic dendrometer data (Deslauriers et al., 2011). The daily SRI was determined by calculating the difference between the maximum values from two consecutive days. The cumulative SRI on a given day was determined by calculating the sum of all daily SRI values before that date.
To assess the dynamics of SRI and RWI, we fitted the cumulative SRI and RWI using a modified Gompertz function defined using the NonLINear regression (NLIN) function provided by the SAS software (with the Marquart iterative option):
| $ y = {y_0} + A\exp \left( { - {{\rm{e}}^{\beta - kt}}} \right) $ | (1) |
where y is the cumulative SRI (or RWI) at time t (determined as the day of year, DOY); A is the upper horizontal asymptote parameter, and represents the final value of cumulative SRI (or RWI) reached at the end of the growing season; y0 is the lower asymptote; β is a time axis placement parameter that reflects the choice of the origin time; and k is a growth-rate parameter that determines the spread of the curve along the time axis. Consequently, A corresponds to the total seasonal growth and (yt-yt-1) corresponds to the daily growth between days t-1 and t. The time (DOY) when maximum growth increment was achieved can be calculated as β/k. The lower asymptote (y0) was used to characterize the complete seasonal growth pattern (April-October) and to avoid arbitrary choices in the initial settings of the dendrometer, because even in absence of cell activity and stem growth, stem diameter variations occur due to day-to-day changes in plant water storage (Duchesne et al., 2012; Wang et al., 2015). Thus, we did not set y0=0 when we modeled the dynamics of SRI, and set y0=0 when we modeled the RWI data.
2.4 Relationship between environmental factors and growthThe growth period between the initiation of growth and the cessation of tree ring formation was used to assess the relationships between environmental factors and growth. The initiation and cessation of tree ring growth are unambiguous in the microcores. However, for the dendrometer data, no unambiguous date of growth initiation or cessation can be determined. In previous studies, some researchers used the date when the dendrometer data permanently exceeded the initial value at the beginning of May (Deslauriers et al., 2003a; Maekinen et al., 2008), or the date when the modeled daily growth values exceeded a certain constant value (Duchesne et al., 2012; Xiao et al., 2012). To account for the short period of data collected before the growth initiation date detected in the microcores, we defined the dates of growth initiation and cessation as the date when the modeled dendrometer daily growth became greater than and less than 5 μm/d, respectively. The date of maximum growth was determined using the modified Gompertz model, as described previously.
The relationships between environmental factors and growth were investigated using correlation analysis between daily SRI indices, weekly SRI indices, and weekly RWI indices and the corresponding daily or weekly environmental variables. The weekly SRI indices equaled the cumulative daily SRI indices for a week, which corresponded to the microcores sampling intervals. However, the ranges of daily and weekly data may be disturbed by gaps in the data. To remove endogenous growth trends, SRI indices and RWI indices were measured using a growth index that equaled the measured value divided by the value predicted by the Gompertz model.
We used correlation coefficients to evaluate the relationships between SRI indices and RWI indices and the environmental variables. The climatic and hydrologic variables included in our analysis were the mean daily air temperature (T), maximum air temperature (Tmax), minimum air temperature (Tmin), relative mean humidity (RH), precipitation (Precip), and sunshine duration (D) based on data from the national weather station at Ejina Banner (Figure 1). The daily groundwater depth (GD) was the average of the observed data for a 24-hour period. We used Kolmogorov-Smirnov tests for normality before deciding which correlation coefficient to use. We calculated Spearman's correlation coefficient (ρ) for data that was not normally distributed and Pearson's correlation coefficient (r) for normally distributed data. These variables were summed (Precip) or averaged (T, Tmax, Tmin, RH, GD, D) at weekly intervals for the weekly data analysis.
3 Results 3.1 Climate and hydrological conditions during the growing seasonDuring the study year, the 10-day mean temperature was greater than 5 °C by early March, and then increased to values greater than 10 °C in early April. The mean daily temperature reached its highest value (32.1°C) on 3 July 2013 (DOY 184; Figure 3). The annual precipitation totaled 34.2 mm, which is near the average value (37.2 mm) from 1957 to 2014. In this year, 24.7 mm of precipitation (72%) fell in June and July. The daily maximum precipitation during these two months was 8.4 mm, with all other rainfall events less than 5 mm. From May 1 to September 30 (DOY 121 to 273), the depth to groundwater averaged 1.9 m at the study site. However, the groundwater depth increased constantly during the growing season due to a combination of evaporation and transpiration, and no runoff reached the site until September 11 as a result of seasonal drying of the Heihe River, which is a desert river that often stops flowing in its lower reaches.
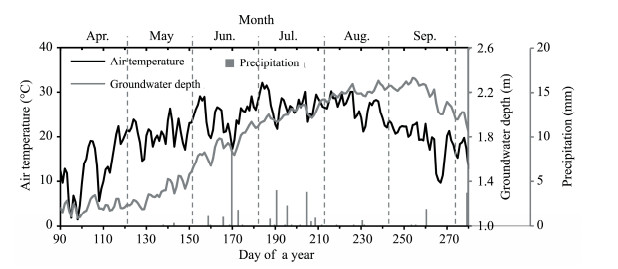
|
| Figure 3 Daily total precipitation, mean air temperature, and mean groundwater depth from DOY (day of year) 90 (March 31) to DOY 280 (October 7) during 2013 |
The cambium consisted of radially flattened cambial cells with thin and non-lignified primary cell walls that stained blue (Figures 2a-d). The morphology of the cells and the number of cell layers in the cambium varied throughout the year. The cambial reactivation had begun by April 21 (DOY 111, Figure 2a), and the cells in the cambium formed 4 to 7 layers. The number of cell layers increased to about 10 by May 5 (DOY 125), after which the number of cambial cells decreased (Figure 4). Between July 7 (DOY 188) and July 14 (DOY 195), cambial division ceased and the cambium contained 2 to 5 cell layers thereafter (Figure 4).
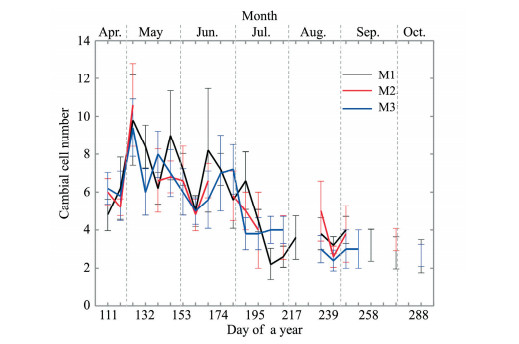
|
| Figure 4 Changes in the number of cambial cell layers in P. euphratica for the three trees measured by means of microcoring (M1, M2, and M3). Values represent means±standard deviations (n=5). Some values could not be determined because of a loss of data during the sampling or laboratory work |
The xylem in P. euphratica consists of vessels, fibers, and parenchyma (Figure 2a). This species has diffuse-porous wood; that is, the vessels in the early wood zone have a large diameter, which gradually but not obviously decreases in size as vessels enter the latewood zone (Figure 2e). Vessels were produced via cell divisions in the cambial zone. Xylem differentiation occurred in two phases: vessel enlargement and wall thickening. During enlargement, vessels turn blue after staining (Figure 2). During the wall thickening phase, the staining of vessels walls changes from blue to red under normal light due to an increase in lignin deposition.
The initiation date for vessel enlargement detected from the microcores of the three trees in 2013 were all on May 5 (DOY 125), but the cessation date for vessel division ranged from July 7 (DOY 188) to July 14 (DOY 195). The duration of the period of vessel enlargement therefore ranged from 63 to 70 days in 2013. The date of the end of tree ring formation ranged from August 5 (DOY 217) to August 27 (DOY 239). The duration of tree ring formation therefore ranged from 92 to 114 days.
Based on the microcores data, the coefficient of determination for the nonlinear Gompertz function regressions of ring-width as a function of time ranged between 0.957 and 0.984 (Table 1; Figure 5). Seasonal growth indicated by the models ranged from 2.809 to 3.616 mm (Table 1). The daily maximum growth rate calculated by the models ranged from 53 to 72 μm/d. The date of maximum growth ranged from June 1 (DOY 152) to June 5 (DOY 156).
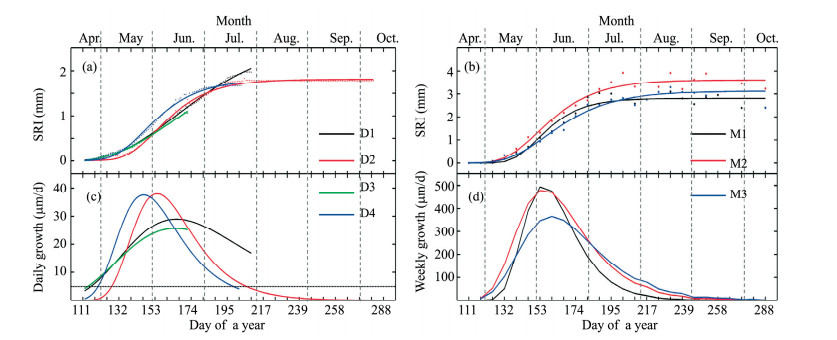
|
| Figure 5 (a) Observed and modeled stem radius increments (SRI) for the trees measured using a dendrometer (D1 to D4) and (b) ring width increase (RWI) measured by means of microcoring (M1 to M3); (c, d) the associated growth rates. Values are for the seven P. euphratica trees observed during the 2013 growing season. Some parts of the SRI series could not be determined robustly because some of the dendrometer data was lost |
| Tree | Start of data (DOY) |
End of data (DOY) |
y0 | A | P | k | R2 | Date of maximum growth (DOY) |
Maximum daily growth (μm/d) |
Seasonal growth (mm) |
Initiation of tree ring formation (DOY) |
End of vessel division (DOY) |
Cessation of tree ring formation (DOY) |
| D1 | 111 | 209 | -0.01 | 2.73 | 4.75 | 0.03 | 0.998 | 165 | 29 | - | 116 | - | - |
| D2 | 111 | 280 | 0.01 | 1.80 | 8.80 | 0.06 | 0.997 | 154 | 38 | 1.805 | 128 | - | 207 |
| D3 | 111 | 172 | -0.03 | 2.55 | 4.49 | 0.03 | 0.998 | 164 | 26 | - | 114 | - | - |
| D4 | 112 | 203 | 0.01 | 1.80 | 8.35 | 0.06 | 0.995 | 147 | 38 | 1.797 | 121 | - | 200 |
| M1 | 111 | 258 | 0 | 2.81 | 10.58 | 0.07 | 0.957 | 152 | 72 | 2.809 | 125 | 188 | 217 |
| M2 | 111 | 258 | 0 | 3.62 | 7.91 | 0.05 | 0.971 | 152 | 69 | 3.616 | 125 | 195 | 224 |
| M3 | 111 | 258 | 0 | 3.13 | 7.13 | 0.05 | 0.984 | 156 | 53 | 3.125 | 125 | 188 | 239 |
| Start and end of data DOY (day of year) refer 10 the original datasets. y0, A, β, and k are the parameters of the regression based on a modified Gompertz function, and R2 is the coefficient of determination of the regression. The seasonal growth, initiation and cessation of tree ring, and maximum daily growth of dendrometer data were calculated from the regression model. The phenology of tree ring formation is described using the grewth initiation date, end of vessel division, and cessation of tree ring formation, which was detected using the dendrometer data or the microcores. Trees D1 to D4 were measured using a dendrometer and trees M1 to M3 were measured using microcores. (-:the data could not be determined robustly because of a lack of dendrometer data). | |||||||||||||
There were only two dendrometer datasets that covered the entire stem radial increment period and could be fully modeled using the Gompertz function. The dates of growth cessation of D1 and D3 could not be detected. The initiation of the stem radial growth detected from the regression models ranged from April 24 (DOY 114) to May 8 (DOY 128), and the dates of growth cessation ranged from July 19 (DOY 200) to July 27 (DOY 207) (Table 1; Figures 5c, d). The lengths of the period of stem radial increment were 79 days for both datasets. The coefficients of determination for the modified Gompertz models for seasonal stem radial increments ranged between 0.995 and 0.998. Total seasonal growth indicated by the models ranged from 1.797 to 1.805 mm (Table 1). The maximum growth rate calculated by the models ranged from 26 to 38 μm/d. The date of maximum growth ranged from May 27 (DOY 147) to June 14 (DOY 165).
3.4 Correlations between growth and environmental factorsTable 2 summarizes the correlations between the environmental factors and weekly RWI indices (trees D1 to D4), weekly SRI indices (trees M1 to M3), and daily SRI indices (trees D1 to D4) during the growth period. Most correlations between daily SRI indices and environmental factors were negative, and the correlations were most often statistically significant for temperature (particularly Tmax) and depth to groundwater. There was no significant correlation between precipitation or relative humidity and daily SRI indices. Sunshine duration was significantly negatively correlated with daily SRI indices for tree D1. All the correlations between groundwater depth and daily SRI indices were negative, and two were significant.
| Parameter | SRI | RWI | ||||||
| D1 | D2 | D3 | D4 | M1 | M2 | M3 | ||
| Weekly (Pearson's r) | ||||||||
| Precip | -0.24 | -0.27 | -0.28 | -0.27 | -0.23 | -0.25 | -0.19 | |
| D | 0.11 | 0.06 | 0.18 | 0.17 | 0.06 | 0.04 | -0.11 | |
| RH | -0.28 | -0.33 | -0.11 | -0.34 | -0.31 | -0.35 | -0.31 | |
| T | -0.27 | -0.38 | -0.14 | -0.57* | -0.34 | -0.42 | -0.48* | |
| Tmin | -0.35 | -0.50 | -0.30 | -0.68* | -0.47 | -0.56* | -0.51* | |
| Tmax | -0.24 | -0.34 | -0.08 | -0.51 | -0.30 | -0.37 | -0.49* | |
| GD | -0.44 | -0.55* | -0.50 | -0.60* | -0.59* | -0.56* | -0.47* | |
| Daily (Spearman's ρ) | ||||||||
| Precip | 0.03 | 0.15 | 0.02 | -0.07 | ||||
| D | -0.26* | -0.13 | -0.19 | -0.21 | ||||
| RH | 0.05 | -0.01 | 0.05 | 0.02 | ||||
| T | -0.25* | -0.46** | -0.18 | -0.20 | ||||
| Tmin | -0.15 | -0.36** | 0.04 | -0.10 | ||||
| Tmax | -0.27** | -0.42** | -0.27 | -0.24* | ||||
| GD | -0.21* | -0.21 | -0.26 | -0.28* | ||||
| * significant at P < 0.05; ** significant at P < 0.01. | ||||||||
For the weekly SRI indices and RWI indices, there was no significant correlation with precipitation, sunshine duration, or relative humidity (Table 2). All of the correlations between temperature variables and weekly SRI indices and RWI indices were negative, but only 5 of the 21 coefficients were significant. The negative correlations between groundwater depth and weekly SRI indices and RWI indices were generally stronger than the other correlations, and were significant for five of the seven trees (excluding D1 and D3).
4 Discussion 4.1 Comparison between microcoring and dendrometer measurementsThe microcoring method calculated longer growth duration than the dendrometer method for 2013, mainly due to the earlier cessation date in the latter method. Although the dendrometers show that the stem radius increase began earlier than the microcoring method (by about five days, on average; Table 1), the dendrometers show an earlier cessation of the stem radius increase (by about 23 days, on average). The earlier growth initiation detected by the dendrometers in 2013 was also found in a comparison of these two methods for Norway spruce (Picea abies (L.) Karst.) and Scots pine (Pinus sylvestris L.) in Finland (Maekinen et al., 2008). There are two likely explanations for the earlier start. First, water uptake around the time of growth initiation will result in an increase of the stem radius without the formation of new xylem cells (Downes et al., 1999; Zweifel et al., 2001). Second, cambial reactivation could contribute greatly to the stem radius change. Figure 2 shows that on April 21, 2013 (DOY 111), the cambial reactivation led to an average 99 μm increase in the stem radius before vessel division began. On May 12 (DOY 132), one week after the start of vessel division, cambial cell width averaged 156 μm, which is approximately the same as the ring width (Figure 2b). The influence of the cambial zone could not be ignored even during the period of maximum growth (Figure 2c). The lignification process after the cessation of vessel division could lead to the detection of an earlier cessation of growth by the dendrometers, because the cell wall thickness adds little to the ring width increment and the change in stem radius. Thus, for this poplar species, it is difficult to use dendrometers to exactly determine the actual period of tree ring formation. However, the dendrometers detected the timing of maximum growth during the growing season as well as the microcoring method (Table 1).
The strongest (negative) correlation coefficients between weekly SRI indices and RWI indices and environmental factors were the same in both methods, indicating that groundwater depth was the major limiting factor for the xylem growth of P. euphratica during the growth period (Table 2). Air temperature played a less important role. Our findings that groundwater depth was the most important limiting factor for stem radial growth of P. euphratica differs from previous studies in temperate mountain and boreal coniferous species, which suggested that temperature (Rossi et al., 2008), precipitation (Ren et al., 2015), or moisture availability (Jiang et al., 2014) were more important. Studies based on isotope hydrology support the present result by indicating that the main water source for P. euphratica is soil water or groundwater (Zhao et al., 2008; Yin et al., 2012; Si et al., 2014), and the soil water in riparian forests of our study area is mainly recharged by groundwater (Xi, 2009; Si et al., 2014). Recent water-relations research also suggests that many trees or shrubs of the riparian zone from the desert regions of North America use groundwater rather than stream water (Smith et al., 1998; Scott et al., 2000), indicating that these trees or shrubs may be sensitive to groundwater availability in riparian corridors (Singer et al., 2014). Precipitation during the 2013 growing season (34.2 mm) was insufficient to contribute greatly to tree growth and the small rainfall events (all except one were < 5 mm; Figure 3) suggest that all or most of the precipitation evaporates before it can reach significant depths in the soil.
The correlations between daily SRI indices and environmental factors differed from those for weekly SRI indices and RWI indices. For daily SRI indices, both the temperature and groundwater depth were limiting factors. This was especially true for the maximum temperature, of which five of the eight negative correlations were significant. This indicates that temperature is one of the major factors that affect the high-frequency (daily) SRI fluctuations. However, because the daily measurements are more sensitive to fluctuations in water storage, weekly SRI datasets (which average out these fluctuations) more accurately describe the volume growth. As the dendrometer measurements provided a time series that simultaneously captures the diurnal rhythms of water storage fluctuations (Offenthaler et al., 2001; De Schepper et al., 2012) and tree growth (Downes et al., 1999; Xiao et al., 2014a, b), it is very difficult to separate xylem growth from changes that result from changes in water storage, particularly at short timescales (Chan et al., 2016). Furthermore, air temperature strongly affects the daily sap flow (Si et al., 2007), which in turn affects stem water storage. Although temperature always affects the photosynthesis of P. euphratica (Zhou et al., 2010), previous researchers concluded that only extremely high temperatures ( > 40 °C) could significantly reduce the photosynthesis of this species (Zhou et al., 2010; Wang et al., 2011), which is a heat-tolerant plant (Ferreira et al., 2006). In our study, the extreme high temperature was 39.1 °C on July 5 (DOY 186). The favorable daily mean temperature (24.8 °C, with a range from 14.6 °C to 32.1 °C from May 1 to August 31) would therefore not limit tree growth.
4.2 Xylem formation patternsThe intra-annual stem radial growth and tree ring formation trends observed in P. euphratica were similar: both show a positive exponential growth phase followed by a phase in which the growth rate decreased, resulting in a sigmoid pattern (Figure 5). This is the same pattern reported for a range of geographical locations and species (Fritts, 1976; Deslauriers et al., 2003a; Schmitt et al., 2004; Wang et al., 2015), and is typical of a deterministic growth process (Klingenberg, 1998). Tree ring formation at breast height in P. euphratica trees started on May 5 (DOY 125). The average daily temperature on May 5 was 23.4 °C (Figure 3), and the minimum temperature was 17.2 °C. This minimum temperature is much higher than the values reported in previous studies in regions with a cold climate (Deslauriers et al., 2008; Rossi et al., 2008), with values of 4 to 5 °C. This early start of growth may have resulted from internal (physiological) responses to the higher temperature, as the suitable temperature for photosynthesis of P. euphratica ranged from about 20 to 40 °C in previous research (Zhu et al., 2011; Cao et al., 2012). This is relatively high compared with the six coniferous species studied by Kattge and Knorr (2007), who found values ranging between 14 and 25 °C.
The rate of cell division was low during the first weeks after cambial reactivation, and maximum cell production occurred around the beginning of June (Figure 5). This is earlier than in previous studies, which reported that the maximum radial growth of seven conifers (Abies alba, Larix decidua, Picea abies, Picea mariana, Pinus cembra, Pinus sylvestris, Pinus uncinata) at the timberline occurred around the summer solstice (June 21), when the photoperiod was longest (Rossi et al., 2006c) or the temperature was highest (Maekinen et al., 2003). Because the sunshine duration in our study area during the growing season was high (with an average value of 9.9 hours from May 1 to August 31), and it did not constrain tree growth. The ongoing increase of groundwater depth throughout the growing season (by about 1.1 m from May 1 to August 31) due to a combination of evaporation and transpiration could be the major limiting factor. Meanwhile, the cessation date for vessel division based on microcore data ranged from early August (DOY 217) to late August (DOY 239), which is similar to the results of previous studies (Deslauriers et al., 2003b; González-González et al., 2013; Ren et al., 2015).
4.3 Implications for dendrochronology studies and riparian forest managementPrevious annual-scale dendrochronology studies in extremely arid areas revealed different growth response of P. euphratica to environmental variables, such as river runoff (Liu et al., 2007a) and groundwater depth (Sun et al., 2006). In our study, we found significantly negative correlations between groundwater depth and stem radial growth during the growth period (Table 2), but we cannot exclude the effects of river runoff on radial growth. Because the discontinuous runoff is the only major source of surface water in the Ejina Oasis, it plays a vital role in maintenance of the local groundwater levels by recharging alluvial aquifers (Su et al., 2009; Xi et al., 2010; Wang et al., 2013). This is also supported by the use of riparian trees for streamflow reconstruction in previous research (Liu et al., 2007b; Meko et al., 2015), which found that soil moisture in the root zone of the riparian trees is directly influenced by fluctuations in stream flow. In our study, there was no surface runoff during the growing season, so the problem of how to quantify the relationship between the continuous intra-annual radial growth and the ephemeral or intermittent runoff must be solved in future research. Because many dendroclimatological studies have shown a significant correlation between tree radial growth and environmental conditions before the growing season (Begum et al., 2013; Scott St, 2014; Yang et al., 2014), we will also need to consider how to evaluate the effects of climatic or hydrologic factors before the growing season on radial growth during the growing season in our future research.
May, June, and July are the main periods of tree-ring formation, as indicated by both the dendrometer data and microcores analysis (Figure 5). Thus, to sustain healthy growth of riparian forests that contain P. euphratica, a suitable groundwater depth must be maintained, particularly during the spring and summer. However, as agricultural water use in the middle reaches of the Heihe River is also concentrated during this period, there is a conflict between agricultural and ecological water uses. To mitigate these conflicts, it will be necessary to carefully consider the optimal time for ecological water diversions to sustain the riparian forests in the lower reaches of the river basin.
5 ConclusionsIn this study, we measured the intra-annual variations in stem radial growth, tree ring formation, and cambial activity of mature P. euphratica trees during the 2013 growing season in a riparian forest in the Ejina Oasis, an extremely arid area of northwestern China. The initiation of vessel enlargement occurred in early May, and the maximum rate of cell production occurred in early June. Cell division ceased from early to mid-July. The cambial cell layer reached its maximum width on May 5. Our comparison of the dendrometer and microcoring methods show an earlier initiation and later cessation of growth in the dendrometer data, although the times of maximum growth were similar. Based on the results of our correlation analysis, groundwater depth had the strongest effect on tree ring growth. The weekly stem radial increment datasets conveyed information on xylem growth more accurately than the daily datasets. Based on the major period of wood formation by P. euphratica, it will be necessary to carefully consider the optimal timing of seasonal ecological water diversions within the Heihe River Basin.
Acknowledgments:This work was supported by the National Key Research and Development Program of China (2016YFC0501001), National Natural Science Foundation of China (No. 91125026, No. 41471082) and the STS project of the Chinese Academy of Sciences (KFJ-EW-STS-00502).
| Allan JD, Erickson DL, Fay J, 1997. The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biology, 37(1): 149–161. DOI: 10.1046/j.1365-2427.1997.d01-546.x |
| Bai Y, Xu HL, Zhang QQ, et al, 2015. Evaluation on ecological water requirement in the lower reaches of Tarim River based on groundwater restoration. Acta Ecologica Sinica, 35(3): 630–640. |
| Begum S, Nakaba S, Yamagishi Y, et al, 2013. Regulation of cambial activity in relation to environmental conditions:understanding the role of temperature in wood formation of trees. Physiologia Plantarum, 147(1): 46–54. DOI: 10.1111/j.1399-3054.2012.01663.x |
| Cao SK, Feng Q, Si JH, et al, 2012. Relationships of photosynthesis and transpiration of Populus euphratica with their affecting factors. Journal of Arid Land Resources and Environment, 26(4): 155–159. |
| Chan T, Hölttä T, Berninger F, et al, 2016. Separating water-potential induced swelling and shrinking from measured radial stem variations reveals a cambial growth and osmotic concentration signal. Plant, Cell & Environment, 39(2): 233–244. DOI: 10.1111/pce.12541 |
| Cheng GD, Xiao HL, Zhao WZ, et al, 2009. Study on the Integrated Management of the Water-Ecology-Economy System of Heihe River Basin. Beijing, China: Science Press. |
| De Schepper V, van Dusschoten D, Copini P, et al, 2012. MRI links stem water content to stem diameter variations in transpiring trees. Journal of Experimental Botany, 63(7): 2645–2653. DOI: 10.1093/jxb/err445 |
| Deslauriers A, Morin H, Urbinati C, et al, 2003a. Daily weather response of balsam fir (Abies balsamea (L. ) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees, 17(6): 477–484. DOI: 10.1007/s00468-003-0260-4 |
| Deslauriers A, Morin H, Begin Y, 2003b. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Canadian Journal of Forest Research, 33(2): 190–200. DOI: 10.1139/X02-178 |
| Deslauriers A, Rossi S, Anfodillo T, et al, 2008. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiology, 28(6): 863–871. DOI: 10.1093/treephys/28.6.863 |
| Deslauriers A, Rossi S, Turcotte A, et al, 2011. A three-step procedure in SAS to analyze the time series from automatic dendrometers. Dendrochronologia, 29(3): 151–161. DOI: 10.1016/j.dendro.2011.01.008 |
| Downes G, Beadle C, Worledge D, 1999. Daily stem growth patterns in irrigated Eucalyptus globulus and E. nitens in relation to climate. Trees, 14(2): 102–111. DOI: 10.1007/PL00009752 |
| Drew DM, Downes GM, 2009. The use of precision dendrometers in research on daily stem size and wood property variation:a review. Dendrochronologia, 27(2): 159–172. DOI: 10.1016/j.dendro.2009.06.008 |
| Duchesne L, Houle D, D'Orangeville L, 2012. Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Québec, Canada. Agricultural and Forest Meteorology, 162-163: 108–114. DOI: 10.1016/j.agrformet.2012.04.016 |
| Edmondson J, Friedman J, Meko D, et al, 2014. Dendroclimatic potential of plains cottonwood (Populus deltoides subsp. monilifera) from the Northern Great Plains, USA. Tree-Ring Research, 70(1): 21–30. DOI: 10.3959/1536-1098-70.1.21 |
| Ferreira S, Hjernö K, Larsen M, et al, 2006. Proteome profiling of Populus euphratica Oliv. upon heat stress. Annals of Botany, 98(2): 361–377. DOI: 10.1093/aob/mcl106 |
| Fritts HC, 1976. Growth and structure. In:Fritts HC (ed). Tree Rings and Climate. London, UK:Academic Press Inc., pp. 70-112. |
| González-González BD, García-González L, Vázquez-Ruiz RA, 2013. Comparative cambial dynamics and phenology of Quercus robur L. and Q. pyrenaica Willd. in an Atlantic forest of the northwestern Iberian Peninsula. Trees, 27: 1571–1585. DOI: 10.1007/s00468-013-0905-x |
| Gričar J, Zupančič M, Čufar K, et al, 2006. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Annals of Botany, 97(6): 943–951. DOI: 10.1093/aob/mcl050 |
| Jiang Y, Wang BQ, Dong MY, et al, 2014. Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of North China. Trees, 29(1): 87–96. DOI: 10.1007/s00468-014-1089-8 |
| Kattge J, Knorr W, 2007. Temperature acclimation in a biochemical model of photosynthesis:a reanalysis of data from 36 species. Plant, Cell & Environment, 30(9): 1176–1190. DOI: 10.1111/j.1365-3040.2007.01690.x |
| Klingenberg CP, 1998. Heterochrony and allometry:the analysis of evolutionary change in ontogeny. Biological Reviews of the Cambridge Philosophical Society, 73(01): 79–123. DOI: 10.1017/S000632319800512X |
| Lite SJ, Stromberg JC, 2005. Surface water and ground-water thresholds for maintaining Populus-Salix forests, San Pedro River, Arizona. Biological Conservation, 125(2): 153–167. DOI: 10.1016/j.biocon.2005.01.020 |
| Liu PX, Chen FH, Jin LY, et al, 2007b. About 100-year reconstruction of spring stream flow based on tree ring in the lower reaches of Heihe River. Arid Land Geography, 30(5): 696–700. |
| Liu PX, Peng JF, Chen FH, 2007a. Hydrological response of Populus euphratica Olve:radial growth in Ejinaa Banner, Inner Mongolia. Journal of Integrative Plant Biology, 49(1): 150–156. DOI: 10.1111/j.1672-9072.2007.00425.x |
| Maekinen H, Noejd P, Sanarpaeae P, 2003. Seasonal changes in stem radius and production of new tracheids in Norway spruce. Tree Physiology, 23: 959–968. DOI: 10.1093/treephys/23.14.959 |
| Maekinen H, Seo JW, Noejd P, et al, 2008. Seasonal dynamics of wood formation:a comparison between pinning, microcoring and dendrometer measurements. European Journal of Forest Research, 127(3): 235–245. DOI: 10.1007/s10342-007-0199-x |
| Meko DM, Friedman JM, Touchan R, et al, 2015. Alternative standardization approaches to improving streamflow reconstructions with ring-width indices of riparian trees. The Holocene, 25(7): 1093–1101. DOI: 10.1177/0959683615580181 |
| Nilsson C, Berggren K, 2000. Alterations of riparian ecosystems caused by river regulation. BioScience, 50(9): 783–792. DOI: 10.1641/0006-3568(2000)050[0783:AORECB]2.0.CO |
| Offenthaler I, Hietz P, Richter H, 2001. Wood diameter indicates diurnal and long-term patterns of xylem water potential in Norway spruce. Trees, 15(4): 215–221. DOI: 10.1007/s004680100090 |
| Peng XM, Xiao SC, Cheng GD, et al, 2015. Human activity impacts on the stem radial growth of Populus euphratica riparian forests in China's Ejina Oasis, using tree-ring analysis. Trees,. DOI: 10.1007/s00468-015-1287-z |
| Ren P, Rossi S, Gricar J, et al, 2015. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau?. Annals of Botany, 115(4): 629–639. DOI: 10.1093/aob/mcu259 |
| Rossi S, Deslauriers A, Anfodillo T, 2006a. Assessment of cambial activity and xylogenesis by microsampling tree species:an example at the alpine timberline. IAWA Journal, 27(4): 383–394. DOI: 10.1163/22941932-90000161 |
| Rossi S, Anfodillo T, Menardi R, 2006b. Trephor:a new tool for sampling microcores from tree stems. IAWA Journal, 27(1): 89–97. DOI: 10.1163/22941932-90000139 |
| Rossi S, Deslauriers A, Anfodillo T, et al, 2006c. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytologist, 170(2): 301–310. DOI: 10.1111/j.1469-8137.2006.01660.x |
| Rossi S, Deslauriers A, Anfodillo T, et al, 2007. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia, 152(1): 1–12. DOI: 10.1007/s00442-006-0625-7 |
| Rossi S, Deslauriers A, Griçar J, et al, 2008. Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography, 17(6): 696–707. DOI: 10.1111/j.1466-8238.2008.00417.x |
| Schmitt U, Jalkanen R, Eckstein D, 2004. Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fennica, 38(2): 167–178. DOI: 10.14214/sf.426 |
| Scott ML, Shafroth PB, Auble GT, 1999. Responses of riparian cottonwoods to alluvial water table declines. Environmental Management, 23(3): 347–358. DOI: 10.1007/s002679900191 |
| Scott RL, James Shuttleworth W, Goodrich DC, et al, 2000. The water use of two dominant vegetation communities in a semiarid riparian ecosystem. Agricultural and Forest Meteorology, 105(1-3): 241–256. DOI: 10.1016/S0168-1923(00)00181-7 |
| Scott St. G, 2014. An overview of tree-ring width records across the Northern Hemisphere. Quaternary Science Reviews, 95: 132–150. DOI: 10.1016/j.quascirev.2014.04.029 |
| Sevanto S, HoeLttae T, Holbrook NM, 2011. Effects of the hydraulic coupling between xylem and phloem on diurnal phloem diameter variation. Plant, Cell & Environment, 34(4): 690–703. DOI: 10.1111/j.1365-3040.2011.02275.x |
| Si JH, Feng Q, Cao SK, et al, 2014. Water use sources of desert riparian Populus euphratica forests. Environmental Monitoring and Assessment, 186(9): 5469–5477. DOI: 10.1007/s10661-014-3796-4 |
| Si JH, Feng Q, Xi HY, et al, 2013. Determination of critical period and requirement of ecological water demanded in the Ejina Oasis in lower reaches of Heihe River. Journal of Desert Reseach, 33(2): 560–567. |
| Si JH, Feng Q, Zhang XY, et al, 2007. Sap flow of Populus euphratica in a desert riparian forest in an extreme arid region during the growing season. Journal of Integrative Plant Biology, 49(4): 425–436. DOI: 10.1111/j.1744-7909.2007.00388.x |
| Singer MB, Sargeant CI, Piegay H, et al, 2014. Floodplain ecohydrology:climatic, anthropogenic, and local physical controls on partitioning of water sources to riparian trees. Water Resources Research, 50(5): 4490–4513. DOI: 10.1002/2014WR015581 |
| Singer MB, Stella JC, Dufour S, et al, 2013. Contrasting water-uptake and growth responses to drought in co-occurring riparian tree species. Ecohydrology, 6(3): 402–412. DOI: 10.1002/eco.1283 |
| Smith SD, Devitt DA, Sala A, et al, 1998. Water relations of riparian plants from warm desert regions. Wetlands, 18(4): 687–696. DOI: 10.1007/BF03161683 |
| Speer JH, 2010. Frontiers in dendrochronology. In:Speer JH (ed). Fundamentals of Tree-ring Research. Tucson:The University of Arizona Press, pp. 252-253. |
| Stella JC, Rodríguez-González PM, Dufour S, et al, 2013. Riparian vegetation research in Mediterranean-climate regions:common patterns, ecological processes, and considerations for management. Hydrobiologia, 719(1): 291–315. DOI: 10.007/s10750-012-1304-9 |
| Stromberg JC, 2001. Influence of stream flow regime and temperature on growth rate of the riparian tree, Platanus wrightii, in Arizona. Freshwater Biology, 46(2): 227–239. DOI: 10.1046/j.1365-2427.2001.00651.x |
| Su YH, Zhu G, Feng Q, et al, 2009. Environmental isotopic and hydrochemical study of groundwater in the Ejina Basin, northwest China. Environmental Geology, 58(3): 601–614. DOI: 10.1007/s00254-008-1534-3 |
| Sun JY, Liu Y, Cai QF, et al, 2006. Climatic and hydrological changes of Ejin, Inner Mongolia, China, during the past 233 years record in tree-rings of Populus euphratica. Quaternary Sciences, 26(5): 799–809. |
| Wang HZ, Han L, Xu YL, et al, 2011. Response of chlorophyll fluorescence characteristics of Populus euphratica heteromorphic leaves to high temperature. Acta Ecologica Sinica, 31(9): 2444–2453. |
| Wang P, Yu JJ, Zhang YC, et al, 2013. Groundwater recharge and hydrogeochemical evolution in the Ejina Basin, northwest China. Journal of Hydrology, 476: 72–86. DOI: 10.1016/j.jhydrol.2012.10.049 |
| Wang ZY, Yang B, Deslauriers A, et al, 2015. Intra-annual stem radial increment response of Qilian juniper to temperature and precipitation along an altitudinal gradient in northwestern China. Trees, 29(1): 25–34. DOI: 10.1007/s00468-014-1037-7 |
| Ward JV, Tockner K, Arscott DB, et al, 2002. Riverine landscape diversity. Freshwater Biology, 47(4): 517–539. DOI: 10.1046/j.1365-2427.2002.00893.x |
| Xi HY, 2009. Study on the groundwater dynamics variation and numerical simulation in Ejina Basin. Ph.D. thesis, Cold and Arid Regons of Environmental and Eengineering Research Institute. (in Chinese) |
| Xi HY, Feng Q, Si JH, et al, 2010. Impacts of river recharge on groundwater level and hydrochemistry in the lower reaches of Heihe River Watershed, northwestern China. Hydrogeology Journal, 18: 791–801. DOI: 10.1007/s10040-009-0562-8 |
| Xiao SC, Xiao HL, Peng XM, 2012. Study of seasonal stem radial growth of Populus euphratica in the lower reaches of the Heihe River. Journal of Glaciology and Geocryology, 34(3): 706–712. |
| Xiao SC, Xiao HL, Peng XM, et al, 2014a. Daily and seasonal stem radial activity of Populus euphratica and its association with hydroclimatic factors in the lower reaches of China's Heihe River Basin. Environmental Earth Sciences, 72: 609–621. DOI: 10.1007/s12665-013-2982-y |
| Xiao SC, Xiao HL, Peng XM, et al, 2014b. Intra-annual stem diameter growth of Tamarix ramosissima and association with hydroclimatic factors in the lower reaches of China's Heihe River. Journal of Arid Land, 6(4): 498–510. DOI: 10.1007/s40333-013 |
| Yang B, Qin C, Wang JL, et al, 2014. A 3, 500-year tree-ring record of annual precipitation on the northeastern Tibetan Plateau. Proceedings of the National Academy of Sciences of the United States of America, 111(8): 2903–2908. DOI: 10.1073/pnas.1319238111 |
| Ye ZX, Chen YN, Li W, 2007. Ecological water demand of vegetation based on eco-hydrological Process in the lower reaches of Tarim River. Acta Geoscientica Sinica, 62(6): 451–461. |
| Yin L, Zhao LJ, Ruan YF, et al, 2012. Study of the replenishment sources of typical ecosystems water and dominant plant water in the lower reaches of the Heihe, China. Journal of Glaciology and Geocryology, 34(6): 1478–1486. |
| Zhang JZ, Gou XH, Zhao ZQ, et al, 2013. Improved method of obtaining micro-core paraffin sections in dendroecological research. Chinese Journal of Plant Ecology, 37(10): 972–977. DOI: 10.3724/SP.J.1258.2013.00100.(inChinese) |
| Zhang QB, Li ZS, Liu PX, et al, 2012. On the vulnerability of oasis forest to changing environmental conditions:perspectives from tree rings. Landscape Ecology, 27: 343–353. DOI: 10.1007/s10980-011-9685-0 |
| Zhao LJ, Xiao HL, Cheng GD, et al, 2008. A preliminary study of water sources of riparian plants in the lower reaches of the Heihe Basin. Acta Geoscientica Sinica, 29(6): 709–718. |
| Zhou HH, Chen YN, Li WH, et al, 2010. Photosynthesis of Populus euphratica in relation to groundwater depths and high temperature in arid environment, northwest China. Photosynthetica, 48(2): 257–268. DOI: 10.1007/s11099-010-0032-5 |
| Zhu GF, Li X, Su YH, et al, 2011. Seasonal fluctuations and temperature dependence in photosynthetic parameters and stomatal conductance at the leaf scale of Populus euphratica Oliv. Tree Physiology, 31(2): 178–195. DOI: 10.1093/treephys/tpr005 |
| Zweifel R, Item H, Häsler R, 2000. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees, 15(1): 50–57. DOI: 10.1007/s004680000072 |
| Zweifel R, Item H, Häsler R, 2001. Link between diurnal stem radius changes and tree water relations. Tree Physiology, 21(12-13): 869–877. DOI: 10.1093/treephys/21.12-13.869 |
 2017, 9
2017, 9


