Article Information
- YongQing Luo, XueYong Zhao, JiePing Ding, Tao Wang . 2016.
- Vertical distribution of Artemisia halodendron root system in relation to soil properties in Horqin Sandy Land, NE China
- Sciences in Cold and Arid Regions, 8(5): 411-418
- http://dx.doi.org/10.3724/SP.J.1226.2016.00411
Article History
- Received: March 24, 2016
- Accepted: June 22, 2016
2. Gansu Academy of Environmental Sciences, Lanzhou, Gansu 730020, China;
3. College of Earth and Environmental Sciences, Lanzhou University, Lanzhou, Gansu 730000, China
Artemisia halodendron is a common sub-shrub in Horqin Sandy Land and Hulun Buir grassland of eastern Inner Mongolia, China. It is well adapted to mobile and semi-mobile sand dunes, on account of its high tolerance to drought, wind erosion and sand burial (Liu et al., 2007; Huang et al., 2011) and the height ability for deep water capture (Ma et al., 2010). It propagates via both sexual and vegetative propagation, and is a common species for sand-stabilization projects (Li et al., 2005), particularly in the Horqin region (Su et al., 2005; Zhang et al., 2004, 2005). Once the shrub is established, it grows and propagates rapidly due to its low nutrient requirement and capacity for vegetative propagation, and it adds to soil organic matter not only through growth in below and above ground, but also as a collector of wind-transported dust and litter (Li et al., 2005).
Plant roots are major components in matter exchange and energy influx in terrestrial ecosystems. On a global scale, total root mass is estimated to 6.6×1015 g in deserts, 14×1015 g in temperate grassland, 41×1015 g in woodland and shrub land, 83×1015 g in tropical rainforests, 31×1015 g in tropical seasonal forests and 35×1015 g in boreal forests (Jackson et al., 1997). Dry matter root/shoot ratio is 0.32 in boreal forest, 0.10 in cropland, 1.2 in sclerophyll shrub land, 3.7 in temperate grasslands and 1.2 in deserts (Jackson et al., 1996). The main pathway of matter exchange between plant and soil is root turnover – roots die and decompose into soil organic matter. Dead roots may be much more resistant to decomposition than the above-ground parts and thus contributed more to stable humus formation (Kätterer et al., 2011), especially the fine roots (Matamala et al., 2003; Stover et al., 2010; Finér et al., 2011). In spite of the low biomass of fine roots, rapid turnover increases their contribution to soil carbon (Mainiero et al., 2009). Plant root system contributes to soil structural stability, soil water infiltration and aggregate stability (Baets et al., 2006; Reubens et al., 2007) and thus is a key factor in soil erosion control (Zhou and Shangguan, 2007). Even in the initial steps of soil creation, plant roots are crucial components in the weathering of rocks and minerals (Lambers et al., 2009).
The need for a comprehensive understanding of the processes and patterns of carbon allocation, especially against the background of global climate change has been emphasized (Gill and Jackson, 2000; Norby and Jackson, 2000). In Horqin Sand Land, studies were focused on soil properties, especially for soil organic carbon and nitrogen (Li et al., 2006) and plant variation along the gradient of sandy land restoration (Zuo et al., 2012). However, detailed research on root-soil system's matter allocation in semi-mobile dunes is rare.
It is generally accepted that soil water availability (Sokalska et al., 2009; Schelle et al., 2013) and nitrogen availability (Głąb and Kacorzyk, 2011) are crucial factors for plant root growth and spatial distribution. Also, soil nitrogen is a limiting factor for the ecological restoration of plants in Horqin Sandy Land (Su et al., 2005; Li et al., 2006). Knowledge on plant-soil system nitrogen allocation can supply significant information on nitrogen management and enhance the nitrogen utilization efficiency, which will benefit ecological restoration of degraded systems in Horqin Sandy Land.
Research has focused on C allocation to roots (Matamala et al., 2003; Mendez-Millan et al., 2012), root C input to soil (Wu et al., 2011; Zhao et al., 2011; Tu et al., 2013), nutrient uptake (Devau et al., 2011; Richardson et al., 2011) and root lifespan as well as turnover (Ostonen et al., 2005; Jourdan et al., 2008; Finér et al., 2011). The vertical distribution of A. halodendron roots in sand dunes has been studied earlier (Hansson et al., 1994, 1995; Zhao, 1994; Liu et al., 2003), but the relationship between soil property and root development in the sandy land is still unclear. In this study we focused on (1) vertical distribution of A. halodendron roots, (2) soil properties under the canopy of the shrub and the distribution of carbon and nitrogen in the underground system, and (3) the relationship between root system development and soil property.
2 Material and methods 2.1 Study siteThis study was conducted at the Naiman Desertification Research Station of the Chinese Academy of Sciences (42°58'N, 120°43'E; 360 m a.s.l.), in the eastern part of Inner Mongolia, China. Naiman is located in the southwestern part of the Horqin Sandy Land, which is roughly 400km×200km in size and represents the most desertification-threatened area in Northeastern China (Figure 1). The landscape is characterized by sand dunes alternating with gently undulating interdune areas. The soil is sandy and particularly susceptible to wind erosion (Li et al., 2005). This area belongs to the temperate zone, with a continental semi-arid monsoon climate. Annual mean precipitation is about 366 mm with high seasonal variability. Annual mean potential evaporation is 1, 935 mm. Annual mean temperature is about 6.7 ℃, and the lowest and highest monthly mean temperatures is −12.6 ℃ in January and 24.3 ℃ in July, respectively.
|
| Figure 1 Location of Horqin Sandy Land and Naiman station |
Semi-mobile dunes with the dominant shrubs Salix gordejevii and A. halodendron were selected as the study site. In August 2012, roots of A. halodendron were sampled with a soil auger (diameter 100 mm) at every 10 cm depth to 60 cm. To obtain reliable estimates of root vertical distribution, the soil coring was performed close to mid-size bushes, about 20 cm from the central stem. Nine bushes with similar size were sampled for replicates which resulted in a total of 54 root samples. The canopy ranged from 50 to 60 cm (East–West) × 60 to 70 cm (South–North) and the height ranged from 30 to 45 cm, respectively.
Soil samples from 0 to 60 cm depth were taken halfway from the central stem to the edge of the bush canopy with a soil auger of 28-mm in diameter, three soil cores per plant in a triangle pattern around the main stem. The three subsamples were bulked to a total sample of about 1 kg fresh mass, and this was made under five of the nine plants sampled for roots. In total, 30 soil samples were taken for analysis (5×6). While sampling, soil bulk density was measured using a cutting ring (100 cm3).
2.3 Laboratory measurementsAll root samples were sieved through a 2-mm sieve after air-dried in the shade, and then rinsed with distilled water for three times. Root biomass and necromass were manually separated by color. The necromass, dark in color, mainly included dead roots and epidermal root tissues sloughed off during the growing season. The remaining live roots were scanned at 200 dpi by HP ScanJet 2400C (HP Company, USA) and stored as BMP files. The live root images were analyzed for root length by CIAS 2.0 software (CID-Bioscience, Camas, WA). Samples of both live roots and necromass were dried at 65 ℃ for 48 hours and then weighed. The samples were then milled for carbon and nitrogen content analysis by an automatic elemental analyzer (vario Macro cube, Elementar, Germany).
The soil samples taken under the canopy were sieved at 2 mm and gravel, litter, live and dead roots were removed. Soil texture was measured by the wet sieving method (ISSCAS, 1978). In our former study (Li et al., 2012b), soil samples were separated into three fractions to describe the texture, which were coarse sand (0.1~2.0 mm), fine sand (0.05~0.10 mm) and silt + clay ( < 0.05 mm). Because most fractions (over 95%) in semi-mobile dune soil was coarse sand, in this study we divided the coarse sand into very coarse (0.5~2.0 mm) and coarse (0.1~0.5 mm) sand. Thus, the soil samples were divided into four fractions: very coarse sand (0.5 to 2.0 mm), coarse sand (0.1 to 0.5 mm), fine sand (0.05 to 0.10 mm) and silt+clay ( < 0.05 mm). Soil organic carbon was determined by dichromate oxidation according to Walkley–Black (Nelson and Sommers, 1982). Total nitrogen was measured by the Kjeldahl procedure (UDK140 Automatic Steam Distilling Unit, Automatic Titroline 96, Italy). Soil pH was measured in a 1:2.5 soil:water suspension and electrical conductivity (EC) in a 1:5 soil:water suspension at 25 ℃ (Multiline F/SET-3, Germany).
2.4 Data analysisStatistical analyses were made using SPSS 13.0. Results were generally analyzed by one-way ANOVA with the different layers as factor. Multiple comparisons using the LSD test were done whenever the ANOVA indicated significant differences (P≤0.05). Correlation analysis was conducted by bivariate correlation method. Correlation coefficient was Pearson and two-tails test was used for significance test. Concentrations of C and N were measured and their densities and stocks in soil were calculated as:
| ${D_s} = {C_s} \times BD \times 1000$ | (1) |
| ${S_s} = {D_s} \times T$ | (2) |
where Dsis C or N density (g/m3) in soil, Cs is soil C or N concentration (g/kg), BD is the soil bulk density (g/cm3); Ss is the Stock (g/m2) and T is the soil layer thickness (m).
For live roots and necromass,
| ${D_b} = {C_b} \times {M_a}/100$ | (3) |
| ${S_b} = {D_b} \times T$ | (4) |
where Db is C or N density (g/m3) in live roots and necromass; Cb is C or N concentration (%) in live roots and necromass; Ma is the mass (g/m3) in live roots and necromass; Sb is C or N stock in live roots and necromass, and T is the soil layer thickness (m).
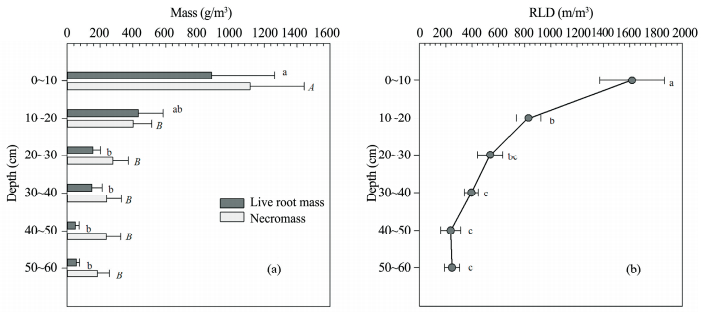
3 Results 3.1 Vertical distribution of rootsThe vertical distribution of A. halodendron root bio-and necromass followed the same general pattern, decreasing with increasing soil depth and mainly located in the surface layer. As much as 76% of the root biomass was in the 0~20 cm layer, while 73% of necromass was above 30 cm depth. The necromass also had a slightly deeper distribution (Figure 2a). In total, more necromass than biomass was found, 409 and 287 g/m3, respectively, and necromass was higher than biomass in each layer except 10~20 cm. Root length density (RLD) also declined with soil depth, following a similar pattern (Figure 2b).
|
| Figure 2 Vertical distribution of root biomass, necromass (a) and root length density (b) of A. halodendron. Capital and lower-case letters indicate significant differences at levels of 1% and 5% respectively |
Carbon concentration was about 44% in both root biomass and the necromass, respectively (Table 1). There were minor, but significant differences between depths in biomass. Moreover, the variability for necromass carbon concentration was higher (ranged from 38.7% to 49.9%), and the carbon concentration in the top layer from 0 to 30 cm was lower than the live root.
| Depth (cm) | C concentration (%) | N concentration (%) | C density (g/m3) | N density (g/m3) | |
| Root biomass | 0~10 | 43.9±0.3bc | 0.82±0.03b | 386.7±167.7a | 7.2±3.0a |
| 10~20 | 43.8±0.4bc | 0.91±0.03b | 188.2±63.8ab | 4.1±1.5ab | |
| 20~30 | 45.9±0.4a | 1.07±0.05a | 70.8±21.0b | 1.7±0.5b | |
| 30~40 | 44.5±0.5ab | 0.86±0.04b | 65.9±26.0b | 1.3±0.5b | |
| 40~50 | 42.9±0.6c | 0.86±0.06b | 21.7±9.8b | 0.5±0.2b | |
| 50~60 | 44.4±0.8ab | 0.91±0.07b | 24.6±9.0b | 0.5±0.2b | |
| Necromass | 0~10 | 38.7±1.3c | 1.43±0.03a | 425.9±118.6a | 15.9±4.7a |
| 10~20 | 41.7±0.5bc | 1.55±0.02a | 165.1±46.2b | 6.1±1.7b | |
| 20~30 | 41.5±1.0bc | 1.40±0.07a | 112.0±37.4b | 3.8±1.3b | |
| 30~40 | 45.1±2.8b | 1.25±0.05b | 102.9±39.1b | 3.0±1.2b | |
| 40~50 | 49.9±1.0a | 1.20±0.03b | 115.9±43.1b | 2.9±1.1b | |
| 50~60 | 44.7±0.9b | 1.17±0.03b | 80.1±31.6b | 2.2±1.0b | |
| Values with different index letters are significantly different at the 5% level. | |||||
Nitrogen concentration of live roots ranged from 0.82% to 1.07%, and the maximum concentration occurred at the 20~30 cm layer. The average nitrogen concentration was higher in the necromass, 1.33% and 0.91% in necromass and live roots, respectively. Nitrogen concentration in necromass was lower in deep soil (below 30 cm) than nearer the surface (0~30 cm).
Carbon and nitrogen density distributions were similar to those of total mass, and they both significantly decreased with soil depth. Furthermore, the live root density was lower than that of necromass.
3.2 Soil propertiesSoil bulk density under A. halodendron was about 1.6 g/cm3 and similar in different soil layers. Mean concentration of total organic carbon in the soil was 0.55 g/kg, and SOC concentration was reduced in deeper layers. SOC concentration in the top layer (0~10 cm) was significantly higher and the variability was also higher. N concentration was similar in all layers, with a mean concentration of 0.066 g/kg.
C concentration decreased with depth and the profile can be divided into 3 layers with respect to SOC: (1) the surface layer at 0~20 cm, where the concentration and density of SOC were both at the highest (SOC density over 1, 000 g/m3and SOC concentration over 0.7 g/kg); (2) subsurface layer at 20~40 cm, where SOC concentration and density were lower than the surface layer; and (3) bottom layer at 40~60 cm, where SOC concentration and density were less than half of that of the surface layer. As a consequence, C/N ratio decreased with depth (Table 2).
| Depth (cm) | Bulk density (g/cm3) | pH | C concentration (g/kg) | N concentration (g/kg) | C density (g/m3) | N density (g/m3) | C/N |
| 0~10 | 1.58±0.01a | 7.3±0.05d | 0.90±0.16a | 0.081±0.010a | 1, 424±267a | 128±17a | 11.1±1.4a |
| 10~20 | 1.58±0.02a | 7.6±0.01c | 0.72±0.09ab | 0.075±0.013a | 1, 133±130ab | 117±18a | 10.3±1.3a |
| 20~30 | 1.61±0.01a | 8.0±0.03a | 0.56±0.09bc | 0.075±0.015a | 897±134bc | 120±24a | 8.0±0.9abc |
| 30~40 | 1.61±0.03a | 8.0±0.04ab | 0.56±0.05bc | 0.060±0.008a | 909±77bc | 97±14a | 9.7±0.6ab |
| 40~50 | 1.57±0.01a | 7.9±0.04b | 0.37±0.07c | 0.064±0.016a | 586±114c | 101±24a | 6.2±1.0c |
| 50~60 | 1.59±0.01a | 8.1±0.08a | 0.34±0.03c | 0.051±0.015a | 534±43c | 81±25a | 8.2±1.6abc |
| Values with different index letters are significantly different at the 5% level. | |||||||
Soil pH was 7.3 in the surface layer (0~10 cm), significantly lower than that in the bottom layer, and increased from 0~10 cm to 20~30 cm layer. Below 30 cm, soil pH varied slightly but significantly (Table 2).
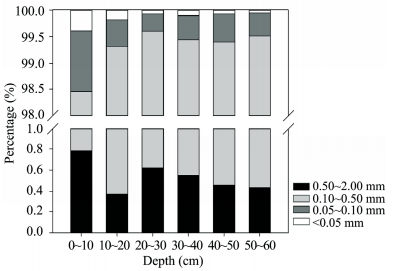
Soil texture differed somewhat between soil layers (Figure 3). Coarse sand (0.1~0.5 mm) was the dominant part (more than 97%) in all soil layers, and increased with soil depth. Fine sand ( < 0.1 mm) was less than 2%, reduced with soil depth, especially for silt + clay ( < 0.05 mm). Very coarse sand was less than 1% and decreased with soil depth.
|
| Figure 3 Soil texture at different soil depth |
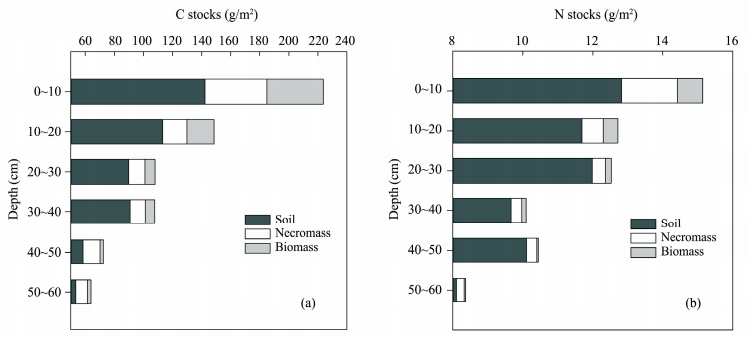
The total amount of C stored at 0~60 cm in the underground system was 724 g/m2 and more than 50% of C was at 0~20 cm depth and declined with soil depth (Figure 4). Total stock of N was 69.2 g/m2and decreased with depth.
|
| Figure 4 Organic carbon and nitrogen belowground allocation |
In terms of component, soil contributed most to the total C and N stocks (Figure 4), more than 60% of C and 80% of N. Compared with root biomass, necromass contributed more to C and N stocks even in this low humus soil.
It is clear from Table 3 that root mass, necromass total mass and RLD were all significantly correlated with C density, especially for the live root mass and the RLD. However, N density showed insignificant relationship with the above parameters.
| Root biomass (g/m3) | Necromass (g/m3) | Total mass (g/m3) | RLD (m/m3) | C density (g/m3) | |
| Necromass (g/m3) | 0.968** | ||||
| Total mass (g/m3) | 0.991** | 0.993** | |||
| RLD (m/m3) | 0.994** | 0.971** | 0.990** | ||
| C density (g/m3) | 0.933** | 0.851* | 0.898* | 0.938** | |
| N density (g/m3) | 0.752 | 0.707 | 0.735 | 0.794 | 0.842* |
| * and ** means correlate significant level is 5% and 1% respectively (2-tailed). Result showed in this table was calculated by the mean value of samples in each soil layer. | |||||
Plant litter is the main source for soil organic matter (SOM) buildup, especially crucial in biotopes such as sandy land, where soil organic carbon and nutrient concentrations are low. Plant litter inputs influence soil microorganism activity and community composition (Doblas-Miranda et al., 2009) and nutrient availability, especially carbon, nitrogen and phosphorus (Tuomi et al., 2009; Manzoni et al., 2012). However, few investigations focus on pattern of plant litter distribution in sandy land. Li et al. (2006) found that litter was concentrated at the top layer (0~10 cm) in all biotopes in Horqin Sandy Land, and 44% of the belowground mass was litter in semi-mobile sand dunes. In that investigation as well as in the present one it is difficult to distinguish between SOM of above-ground vs. below-ground origin. It can be expected that the contribution from above-ground litter is higher in the top layer and adds to the average mass and variability there. In this study, the separation into root biomass, necromass and SOM (excluding live and dead roots) partly resolves this, but without isotope labeling, it is impossible to fully separate SOM into root-or shoot-derived. Thus, the high C and N stocks in the 0~10 cm layer SOM (Figure 4) must have above-ground origin.
Root survival and growth of A. halodendron are affected by competition and soil water availability (Hansson et al., 1994; Huang et al., 2008). For example, root biomass increased significantly in the dry summer season but insignificantly in the wet season in Horqin Sandy Land (Hansson et al., 1995). Root system properties are important for plant establishment and survival in sandy land or desert, and roots of A. halodendron reached 120 cm depth three years after planting (Li et al., 1994), or 90 cm depth in Li et al. (2012a). In this study, root mass decreased with soil depth, and most was allocated in the top layer of the soil, and Zhao (1994) found that roots mainly distributed at 5~30 cm depth in semi-mobile dune. Liu et al. (2003) found that in both fixed and shifting sand land, more than 95% of the roots were at 0~60 cm depth, and most at 0~15 cm. These results were similar to ours; 51.0% and 25.1% of root biomass was at 0~10 and 10~20 cm, respectively.
The relationship between soil property and plant root is complex. Root growth and distribution is influenced by soil properties such as soil texture, soil water and temperature dynamics (Mei et al., 2004). Another important factor is soil nutrient availability, which not only limits the whole plant growth, but also root growth (Geisseler et al., 2009). Nutrient uptake from soil to plant is a key process for plant growth; uptake of water and essential elements such as N, P, K is a key role for plant roots. However, roots contribute to ecosystem building through soil formation by adding large amounts of organic matter to the soil, in many cases more than the contribution from shoots (Janssens et al., 2002; Kätterer et al., 2011). This input is crucial for soil restoration, especially in barren regions such as sandy land. Transport of C from root to soil is mainly via the emission of exudates from live roots (Landi et al., 2006) and the decomposition of dead roots and exfoliation from live root surface (Kätterer et al., 2011). Our study confirms the aforementioned process such that live roots (including biomass and RLD) and necromass (mainly from dead roots and the exfoliation) were all positively related with the C stock in soil (Table 3). Moreover, root exudates from live roots can declined soil pH and create a rhizosphere (Landi et al., 2006; Zhu et al., 2009; Zhou et al., 2011), which also is beneficial for plant growth. In this study, soil pH was lower (Table 2) in top layers with high root biomass and root length density (Figure 2), and this result indicated that live root emission of exudates played an important role in matter exchange in the system between plant and soil in sandy land ecosystems.
In conclusion, the main input of organic carbon and nitrogen to soil is from root process, and the total mass, including live root biomass and necromass, were all positively correlated with C density. However, there was no significant correlation between root bio-or necromass with N density, which probably reflects the N management by the plant – N is a limiting element and N density may be affected by some other factors such as re-adsorption by live plants and leaching or mineralization.
Acknowledgments:This work was financially supported by the National Nature Science Foundation of China (No. 31500369) and the "One Hundred Talent" Program (Y551821001 and Y451H31001) of Chinese Academy of Sciences. We also thank the colleagues of Naiman Desertification Research Station, Chinese Academy of Sciences, for their help in laboratory analysis.
| Andrén O, Zhao X, Liu X, 1994. Climate and litter decomposition in Naiman, Inner Mongolia, China. Ambio, 23: 222–224. |
| Baets SD, Poesen J, Gyssels G, et al, 2006. Effects of grass roots on the erodibility of topsoils during concentrated flow. Geomorphology, 76: 54–67. doi: 10.1016/j.geomorph.2005.10.002 |
| Chang X, Zhao H, Yang C, et al, 2000. Influence of plant species diversity on productivity of sandy grassland in Horqin Region. Chinese Journal of Applied Ecolgy, 11: 395–398. |
| Devau N, Hinsinger P, Cadre EL, et al, 2011. Root-induced processes controlling phosphate availability in soils with contrasted P-fertilized treatments. Plant and Soil, 348: 203–218. doi: 10.1007/s11104-011-0935-3 |
| Doblas-Miranda E, Sánchez-Piñero F, González-Megías A, 2009. Different structuring factors but connected dynamics shape litter and belowground soil macrofaunal food webs. Soil Biology and Biochemistry, 41: 2543–2550. doi: 10.1016/j.soilbio.2009.09.014 |
| Finér L, Ohashi M, Noguchi K, et al, 2011. Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. Forest Ecology and Management, 262: 2008–2023. doi: 10.1016/j.foreco.2011.08.042 |
| Geisseler D, Horwath WR, Doane TA, 2009. Significance of organic nitrogen uptake from plant residues by soil microorganisms as affected by carbon and nitrogen availability. Soil Biology and Biochemistry, 41: 1281–1288. doi: 10.1016/j.soilbio.2009.03.014 |
| Gill RA, Jackson RB, 2000. Global patterns of root turnover for terrestrial ecosystems. New Phytologist, 147: 13–31. doi: 10.1046/j.1469-8137.2000.00681.x |
| Głąb T, Kacorzyk P, 2011. Root distribution and herbage production under different management regimes of mountain grassland. Soil and Tillage Research, 113: 99–104. doi: 10.1016/j.still.2011.02.008 |
| Hansson AC, Zhao A, Andrén O, 1994. Fine-root growth dynamics of two shrubs in semiarid rangeland in Inner Mongolia, China. Ambio, 23: 225–228. |
| Hansson AC, Zhao A, Andrén O, 1995. Fine-root production in degraded vegetation in Horqin Sandy Rangeland in Inner Mongolia, China. Arid Soil Research and Rehabilitation, 9: 1–13. doi: 10.1080/15324989509385869 |
| Huang G, Zhao X, Su Y, et al, 2008. Vertical distribution, biomass, production and turnover of fine roots along a topographical gradient in a sandy shrubland. Plant and Soil, 308: 201–212. doi: 10.1007/s11104-008-9620-6 |
| Huang W, Zhao X, Zhao X, et al, 2011. A combined approach using ISSR and ITS analysis for the characterization of Artemisia halodendron from Horqin sandy land, northern China. Biochemical Systematics and Ecology, 39: 346–351. doi: 10.1016/j.bse.2011.04.011 |
| ISSCAS-Institute of Soil Sciences, Chinese Academy of Sciences, 1978. Physical and Chemical Analysis Methods of Soils. Shanghai: Shanghai Science Technology Press: pp. 7-59. |
| Jackson RB, Canadell J, Ehleringer JR, et al, 1996. A global analysis of root distributions for terrestrial biomes. Oecologia, 108: 389–411. doi: 10.1007/BF00333714 |
| Jackson RB, Mooney HA, Schulze ED, 1997. A global budget for fine root biomass, surface area, and nutrient contents. Pro-ceedings of the National Academy of Science, 94: 7362–7366. doi: 10.1073/pnas.94.14.7362 |
| Janssens IA, Sampson DA, Curiel-Yuste J, et al, 2002. The carbon cost of fine root turnover in a Scots pine forest. Forest Ecology and Management, 168: 231–240. doi: 10.1016/S0378-1127(01)00755-1 |
| Jourdan C, Silva EV, Gonçalves JLM, et al, 2008. Fine root pro-duction and turnover in Brazilian Eucalyptus plantations under contrasting nitrogen fertilization regimes. Forest Ecology and Management, 256: 396–404. doi: 10.1016/j.foreco.2008.04.034 |
| Kätterer T, Bolinder MA, Andrén O, et al, 2011. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agri-culture, Ecosystems and Environment, 141: 184–192. doi: 10.1016/j.agee.2011.02.029 |
| Lambers H, Mougel C, Jaillard B, et al, 2009. Plant-microbe-soil interactions in the rhizosphere:an evolutionary perspective. Plant and Soil, 321: 83–115. doi: 10.1007/s11104-009-0042-x |
| Landi L, Valori F, Ascher J, et al, 2006. Root exudate effects on the bacterial communities, CO2 evolution, nitrogen trans-formations and ATP content of rhizosphere and bulk soils. Soil Biology and Biochemistry, 38: 509–516. doi: 10.1016/j.soilbio.2005.05.021 |
| Li F, Zhang A, Duan S, et al, 2005. Patterns of reproductive allo-cation in Artemisia halodendron inhabiting two contrasting habitats. Acta Oecologica, 28: 57–64. doi: 10.1016/j.actao.2005.02.005 |
| Li J, Liu Z, Li S, et al, 1994. Establishment of artificial vegetation model for Korqin sandy land. Chinese Journal of Applied Ecology, 5(1): 46–51. |
| Li K, Luo Y, Zhang H, et al, 2012a. The relations between root distribution of Artemisia halodendron and soil water in Horqin. Journal of Arid Land Resources and Environment, 26(8): 167–171. |
| Li Y, Zhao H, Zhao X, et al, 2006. Biomass energy, carbon and nitrogen stores in different habitats along a desertification gradient in the semiarid Horqin Sandy Land. Arid Land Research and Management, 20: 43–60. doi: 10.1080/15324980500369285 |
| Li Y, Zhao X, Chen Y, et al, 2012b. Effects of grazing exclusion on carbon sequestration and the associated vegetation and soil characteristics at a semi-arid desertified sandy site in Inner Mongolia, northern China. Canadian Journal of Soil Science, 92(6): 807–819. doi: 10.4141/CJSS2012-030 |
| Liu B, Liu Z, Guan D, 2007. Seedling growth variation in response to sand burial in four Artemisia species from different habitats in the semi-arid dune field. Trees, 22: 41–47. doi: 10.1007/s00468-007-0167-6 |
| Liu S, Piao J, An M, et al, 2003. Distribution dynamics of Artemisia halodendron absorbent roots in different kinds of sandy land. Acta Phytoecologica Sinica, 27(5): 684–689. |
| Ma J, Liu Z, Zeng D, et al, 2010. Aerial seed bank in Artemisia species:how it responds to sand mobility. Trees, 24: 435–441. doi: 10.1007/s00468-010-0411-3 |
| Mainiero R, Kazda M, Haberle KH, et al, 2009. Fine root dynamics of mature European beech (Fagus sylvatica L.) as influenced by elevated ozone concentrations. Environmental Pollution, 157: 2638–2644. doi: 10.1016/j.envpol.2009.05.006 |
| Manzoni S, Piñeiro G, Jackson RB, et al, 2012. Analytical models of soil and litter decomposition:Solutions for mass loss and time-dependent decay rates. Soil Biology and Biochemistry, 50: 66–76. doi: 10.1016/j.soilbio.2012.02.029 |
| Matamala R, Gonzalez-Meler MA, Jastrow JD, et al, 2003. Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science, 302: 1385–1387. doi: 10.1126/science.1089543 |
| Mei L Wang Z, Cheng Y, et al, 2004. A review:factors influencing fine root longevity in forest ecosystems. Chinese Journal of Plant Ecology, 28: 704–710. doi: 10.17521/cjpe.2004.0094 |
| Mendez-Millan M, Dignac MF, Rumpel C, et al, 2012. Contribution of maize root derived C to soil organic carbon throughout an agricultural soil profile assessed by compound specific 13C analysis. Organic Geochemistry, 42: 1502–1511. doi: 10.1016/j.orggeochem.2011.02.008 |
| Nelson DW, Sommers LE, 1982. Total carbon, organic carbon and organic matter. In:Page AL, Miller RH, Keeney DR (eds.). Methods of Soil Analysis, 2nd Edition. American Society of Agronomy, Madison WI, pp. 539-577. Nelson DW, Sommers LE, 1982. Total carbon, organic carbon and organic matter. In:Page AL, Miller RH, Keeney DR (eds.). Methods of Soil Analysis, 2nd Edition. American Society of Agronomy, Madison WI, pp. 539-577. |
| Norby RJ, Jackson RB, 2000. Root dynamics and global change:seeking an ecosystem perspective. New Phytologist, 147: 3–12. doi: 10.1046/j.1469-8137.2000.00676.x |
| Ostonen I, Löhmus K, Pajuste K, 2005. Fine root biomass, pro-duction and its proportion of NPP in a fertile middle-aged Norway spruce forest:Comparison of soil core and ingrowth core methods. Forest Ecology and Management, 212: 264–277. doi: 10.1016/j.foreco.2005.03.064 |
| Reubens B, Poesen J, Danjon F, et al, 2007. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture:a review. Trees, 21(4): 385–402. doi: 10.1007/s00468-007-0132-4 |
| Richardson AE, Lynch JP, Ryan PR, et al, 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil, 349: 121–156. doi: 10.1007/s11104-011-0950-4 |
| Schelle H, Durner W, Iden SC, et al, 2013. Simultaneous estimation of soil hydraulic and root distribution parameters from ly-simeter data by inverse modeling. Procedia Environmental Sciences, 19: 564–573. doi: 10.1016/j.proenv.2013.06.064 |
| Sokalska DI, Haman DZ, Szewczuk A, et al, 2009. Spatial root distribution of mature apple trees under drip irrigation system. Agricultural Water Management, 96: 917–924. doi: 10.1016/j.agwat.2008.12.003 |
| Stover DB, Day FP, Drake BG, et al, 2010. The long-term effects of CO2 enrichment on fine root productivity, mortality, and sur-vivorship in a scrub-oak ecosystem at Kennedy Space Center, Florida, USA. Environmental and Experimental Botany, 69: 214–222. doi: 10.1016/j.envexpbot.2010.03.003 |
| Su Y, Zhang T, Li Y, et al, 2005. Changes in soil properties after establishment of Artemisia halodendron and Caragana mi-crophylla on shifting sand dunes in semiarid Horqin Sandy Land, northern China. Environmental management, 36: 272–281. doi: 10.1007/s00267-004-4083-x |
| Tu L, Hu T, Zhang J, et al, 2013. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biology and Biochemistry, 58: 255–264. doi: 10.1016/j.soilbio.2012.12.005 |
| Tuomi M, Thum T, Järvinen H, et al, 2009. Leaf litter decomposition-Estimates of global variability based on Yasso07 model. Ecological Modelling, 220: 3362–3371. doi: 10.1016/j.ecolmodel.2009.05.016 |
| Wu J, Liu Z, Chen D, et al, 2011. Understory plants can make substantial contributions to soil respiration:Evidence from two subtropical plantations. Soil Biology and Biochemistry, 43: 2355–2357. doi: 10.1016/j.soilbio.2011.07.011 |
| Zhang J, Zhao H, Zhang T, et al, 2005. Community succession along a chronosequence of vegetation restoration on sand dunes in Horqin Sandy Land. Journal of Arid Environments, 62: 555–566. doi: 10.1016/j.jaridenv.2005.01.016 |
| Zhang T, Zhao H, Li S, et al, 2004. A comparison of different measures for stabilizing moving sand dunes in the Horqin Sandy Land of Inner Mongolia, China. Journal of Arid Envi-ronments, 58: 203–214. doi: 10.1016/j.jaridenv.2003.08.003 |
| Zhao A, 1994. Distribution and dynamics of root systems of Artemisia halodendron and Caragana microphylla. Grassland of China, 3: 15–19. |
| Zhao C, Zhao Z, Hong Z, et al, 2011. Contribution of root and rhizosphere respiration of Haloxylon ammodendron to seasonal variation of soil respiration in the Central Asian desert. Quaternary International, 244: 304–309. doi: 10.1016/j.quaint.2010.11.004 |
| Zhou N, Liu P, Wang Z, et al, 2011. The effects of rapeseed root exudates on the forms of aluminum in aluminum stressed rhi-zosphere soil. Crop Protection, 30: 631–636. doi: 10.1016/j.cropro.2011.02.011 |
| Zhou Z, Shangguan Z, 2007. Vertical distribution of fine roots in relation to soil factors in Pinus tabulaeformis Carr. forest of the Loess Plateau of China. Plant and Soil, 291: 119–129. doi: 10.1007/s11104-006-9179-z |
| Zhu Y, Zhang S, Huang H, et al, 2009. Effects of maize root exudates and organic acids on the desorption of phenanthrene from soils. Journal of Environmental Sciences, 21: 920–926. doi: 10.1016/S1001-0742(08)62362-1 |
| Zuo X, Zhao X, Wang S, et al, 2012. Influence of dune stabilization on relationship between plant diversity and productivity in Horqin Sand Land, Northern China. Environmental Earth Sci-ences, 67: 1547–1556. doi: 10.1007/s12665-012-1950-2 |
 2016, 8
2016, 8


