Article Information
- FengLei Feng, Yin Luo Wan, BaoDong Zhi, ZhuSang Ying, ZhuLuo Li, Huang Gang, Liu Hua, Zhang Chen Qi . 2016.
- An investigation of the effects of dust storms on rat lung using HRCT and blood gas analysis
- Sciences in Cold and Arid Regions, 8(4): 319-324
- http://dx.doi.org/10.3724/SP.J.1226.2016.00319
Article History
- Received: January 06, 2016
- Accepted: June 06, 2016
2. Department of Radiation Imaging, the People's Hospital of Gansu Province, Lanzhou, Gansu 730000, China;
3. Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
Sand-dust storms occur when strong winds suspend surface sand and dust, and the resulting visibility is often less than 1 km (Wang et al., 2000). In the last half century, climate change and population increase have led to a greater intensity and frequency of sand-dust storms in China. An increase in associated environmental and health issues has led to heightened public attention. The fine particles transported by sand-dust storms carry many chemical and biological contaminants which can be transported for long distance, causing environmental damage and deleterious health effects (Wang et al., 2010). Fine dust particles (2.5 m or less) can be inhaled directly into the human respiratory tract, causing lung damage. In the spring, sand-dust storms often happen, and the number of patients in hospital suffering from respiratory diseases significantly increases. It is speculatedthat sand-dust storms might induce some acute and chronic respiratory. Non-occupational pneumoconiosis is closely associated with geographic areas prone to sand-dust storms (Huang and Wang, 2001; Meng et al., 2003; Meng et al., 2006). The clinical features are heterogeneous, and they progress acutely or chronically, but the prognosis is very poor.
Health damage caused by sand-dust storms has certain cumulative and latent attack, which threaten the health and lives of the people living in the sandy areas. A multi-stage random survey in Minqin County of Gansu Province, China, showed that the prevalence of non-occupational pneumoconiosis was greater than 5.33‰ (Meng et al., 2008). Some early studies also disclosed that non-occupational pneumoconiosis caused by long-term exposure to dust weather had a high incidence (Hirsch et al., 1974; Fennerty et al., 1983). Most present studies on sand-dust storms are related with agriculture, meteorology, geology, geography and environmental disasters. These have made a lot of progress in the research areas including formation mechanism, transmission pathway, material composition and environmental impact of sand-dust storms. Many studies about sand-dust storms leading to human health hazards focus on the impacts of air particulate contaminants to the health of residents, with few on the direct impact from sand-dust storms, especially scarce on the impact of sand-dust storms to human or animal and its action mechanism. The relationships between sand-dust storms and disease pathophysiology are not very clear. Some present small-scale studies are of case reports and surveys, most of which speculate about the relationship between sand-dust storms and disease only by symptoms and in vitro data, but direct evidence that causing lung disease in real dust conditions was lacking till now.
In this study, we set up an indoor dust-circulating wind tunnel to simulate a natural dust storm environment, and take rats as the experimental objects. It is an internationally pioneering research for qualitatively studying the effect of dust storms on rat lung tissue using HRCT and blood gas analysis. The goal was to answer these questions: a) Can sand-dust storms directly affect the lung function of human? b) What are the mechanisms of sand-dust storms' effect on human lung?
2 Materials and methods 2.1 Ethics statementWistar rat is a kind of medically experimental animal which was widely used in clinical research. The laboratory Wistar rats are provided by the Medical Experimental Animal Centerof the Gansu University of Chinese Medicine. Our experiment with Wistar rats has been authorized by the Ethics Committee of the People's Hospital of Gansu Province.
2.2 Experimental rats' treatmentClean, healthy adult male Wistar rats (weight 160~180 g) were randomly divided into three groups with 40 individuals each. The experimental group was exposed to a simulated dust storm environment in wind tunnel for five hours each day. The second group, as treated control, was exposed to a similar wind tunnel for five hours every day, but no dust was added. The third group, as untreated control, was placed in a standard living environment just outside the wind tunnel in the same room. Rats in these three groups were supplied with same food, water, temperature, humidity, and other living conditions.
2.3 Dust samples and dust storm simulationDusts for experiment are collected from the Alxa Plateau of Inner Mongolia, the source region of dust storm of northern China, by permission of the local land management bureau. The micro morphology of dust particles was observed by scanning electron microscopy (SEM) and the composition of the dust particles was determined by nano-particle size analysis. Results showed that the particle size largely concentrated between 600~1, 100 nm, accounting for about 35%; Si was the most common element (occupying 21.2%), followed by Al (9.52%), Ca (9.01%), Fe (6.92%), Mg (2.42%), Mn (1.75%), and Na (0.73%) (Figure 1).

|
| Figure 1 Characteristics of the dust particles.(a) dust particle size distribution, (b) SEM morphology, (c) composition distribution |
Dust storms are formed in a dust-circulating wind tunnel with cross-sectional area of 600mm×600mm, length of 4 m. Dusts are introduced into the tunnel through the bottom at the entrance of the wind tunnel, experimental wind speed can be adjusted continuously within range of 1~20 m/s. The average ground air dust concentration of TSP was measured to be 9, 000 μg/m3 when stable wind-dust clouds are formed in the wind tunnel, and it can represent the average dust concentration in simulated strong dust storms. The dust concentration was measured using an EPAM-5000 TSP automatic analyzer (SKC Inc, Eighty Four, PA, USA; with a measuring range of 0.001~20.0 mg/m3, an accuracy of ±0.003 mg/m3) at gas flow of 1.0~5.0 L/min, experimental wind speed of 8 m/s. The PM2.5, PM5, PM10 values measured was 0.1697, 0.2441, and 0.5873 mg/m3, respectively.
2.4 MethodsOn the 30th, 60th, 90th, and 120th day after exposures in the dust environment, high-resolution computed tomography (HRCT) and blood gas analysis were performed on randomly selected 10 rats from each group. After weighing, the rats were anesthetized with 10% chloral hydrate (0.5 mL/(100g)) by intraperitoneal injection. A 128-slice spiral CT scan was performed on each rat from the lung apex to the bottom line. The CT scanner (General Electric Company, Fairfield, CT, USA) performed with parameters of 80 kV voltage, 200 mA current, 0.516 screw pitch, and a 0.625 mm layer. After rat lung HRCT examination, blood from the abdominal aorta of each test rat was obtained for blood gas analysis using a model GEM3500 blood gas analyzer (American Experimental Instrument Company, Blood Gas Analyzer, USA). The test data obtained in experiment are statistically analyzed using SPSS v.17.0.
3 Results 3.1 HRCT scanAt each sampling time (the 30th, 60th, 90th, and 120th day after exposures in the dust environment), the lung's HRCT of the treated control group and untreated control group rats were symmetrical, with clear lung texture and good transmittance in both lungs. For the experimental group exposed in dust storm, the lung's HRCT images are shown as follows: on day 30th, the both lung's texture was clear (Figure 2, Table 1); on day 60th, lung's texture of 7 rats exhibited disorder (Figure 3, Table 1). On day 90th, lung's texture of rats exhibited disorder 100%. Patches and a small quantity of ground-glass shadows were visible in the pulmonary fields within the part of rats (Figure 4, Table 1). On day 120th, lung's texture of rats also exhibited disorder 100%. The multiple fiber streaks were exhibited in the pulmonary fields within the part of rats. Ground-glass lesions and patches were significantly increased as compared to that on the day 90th (Figure 5, Table 1).
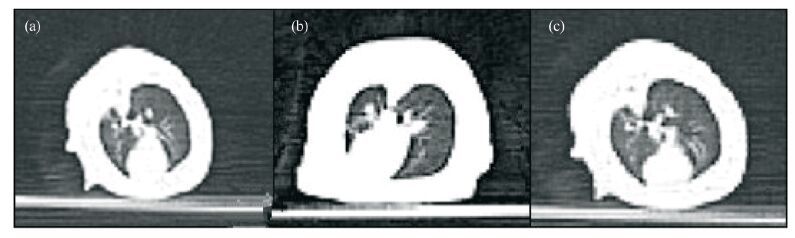
|
| Figure 2 Pulmonary HRCT images at day 30th.(a) untreated control group, (b) treated control group, (c) experimental group |
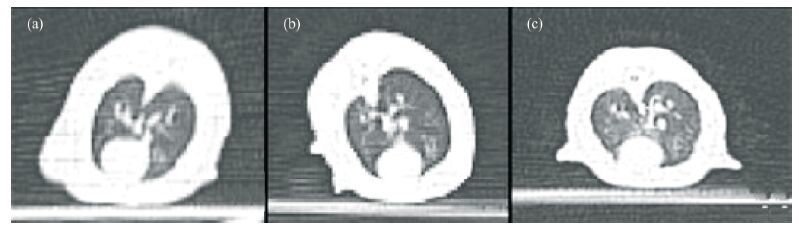
|
| Figure 3 Pulmonary HRCT images at day 60th.(a) untreated control group, (b) treated control group, (c) experimental group |
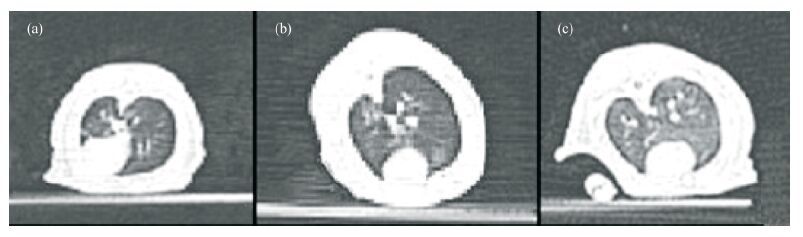
|
| Figure 4 Pulmonary HRCT images at day 90th.(a) untreated control group, (b) treated control group, (c) experimental group |
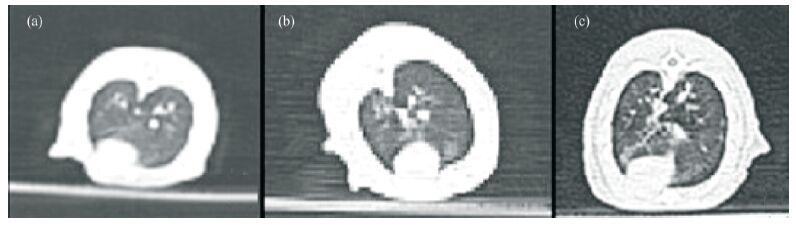
|
| Figure 5 Pulmonary HRCT images at day 120th.(A) untreated control group, (B) treated control group, (C) experimental group |
Statistical analysis of the observed data was performed using SPSS v.17.0. Arterial blood gas parameters were expressed as a mean standard deviation and the test was used to compare independent samples between groups. The comparison of groups at different test times used single factor analysis of variance with P<0.05 when the difference was statistically significant.
Analyzing results showed that no differences in the parameters of blood gas analysis (P>0.05) were evident between the untreated and treated control groups during the study. Additionally, comparisons between the experimental group and two control groups (untreated and treated control) showed no significant differences (P>0.05) in blood gas analysis parameters. Comparisons among the experimental group for all parameters also showed no significant differences (P> 0.05) (Table 1).
| Groups | PaO2(mmHg) | PaCO2(mmHg) | P (A-a) O2(mmHg) |
| Untreated control | |||
| Day 30th | 85.31±6.13 | 34.83±4.56 | 11.16±0.26 |
| Day 60th | 84.48±4.67 | 37.23±6.56 | 9.00±0.78 |
| Day 90th | 83.12±5.17 | 35.46±4.37 | 12.55±0.47 |
| Day 120th | 83.32±6.06 | 34.76±4.45 | 11.23±0.68 |
| Treated control | |||
| Day 30th | 85.12±5.42 | 35.61±3.21 | 10.37±0.42 |
| Day 60th | 85.03±6.12 | 36.71±4.86 | 9.08±0.45 |
| Day 90th | 83.16±6.07 | 35.28±4.24 | 12.74±0.76 |
| Day 120th | 83.37±5.46 | 36.06±3.64 | 11.55±0.47 |
| Experimental group | |||
| Day 30th | 85.12±5.42 | 35.61±3.21 | 10.37±0.42 |
| Day 60th | 82.32±8.19 | 37.82±6.98 | 9.40±0.64 |
| Day 90th | 78.49±8.33 | 38.35±5.47 | 12.75±0.58 |
| Day 120th | 81.78±6.54 | 37.63±6.05 | 11.14±0.31 |
Particulates suspended during sand dust storms can be ingested deep into the airways of humans and animals, possibly disrupting the phagocytosis of macrophages and weakening the immune system, leading to lung disease (Hefflin et al., 1994; Meng et al., 2003). In this study, rat lungs exhibited changes in HRCT images when they are exposed to dust storm over time, and the pulmonary damage degree is positively correlated with the exposure duration (Table 2).
| Groups | Number of rats per test | Lung texture disorder | Patch | Ground-glass shadow | Fiber streak | Times of abnormity |
| Untreated control | ||||||
| Day 30th | 10 | 0 | 0 | 0 | 0 | 0 |
| Day 60th | 10 | 0 | 0 | 0 | 0 | 0 |
| Day 90th | 10 | 0 | 0 | 0 | 0 | 0 |
| Day 120th | 10 | 0 | 0 | 0 | 0 | 0 |
| Treated control | ||||||
| Day 30th | 10 | 0 | 0 | 0 | 0 | 0 |
| Day 60th | 10 | 0 | 0 | 0 | 0 | 0 |
| Day 90th | 10 | 0 | 0 | 0 | 0 | 0 |
| Day 120th | 10 | 0 | 0 | 0 | 0 | 0 |
| Experimental | ||||||
| Day 30th | 10 | 0 | 0 | 0 | 0 | 0 |
| Day 60th | 10 | 7 | 0 | 0 | 7 | |
| Day 90th | 10 | 10 | 4 | 3 | 3 | 20 |
| Day120th | 10 | 10 | 7 | 8 | 7 | 32 |
With increasing exposure, lung destruction became progressively worse, eventually turning into ground-glass and fibrous lesions. A great amount of fine particles will be blown into the air in sand dust storm and inhaled into deep respiratory tract, which affects the phagocytosis capacity of macrophage of alveolus, causing decreased immune function and serious pathological changes in lung tissues. These changes directly cause the reduction in alveolar gas distribution and thickening of the alveolar septa. Previous research (Lei et al., 2015a, b) found that a lot of interstitial lung collagen deposition, the alveolar type Ⅱ cells decrease, lamellar body empties and collagen fiber's formation can cause lung interstitial fibrosis after exposure in the dust environment in several days. In this experiment, HRCT results revealed the lung texture disorder, patches, fiber streaks and ground-glass shadows after exposure in the dust environment 90 and 120 days. These direct HRCT images combined with our previous pathology testing results confirms that the damage of lung tissue was positively correlated with exposure time. HRCT can reflect the pathological process in rat pulmonary interstitial fibrosis. It not only determines whether there is pulmonary fibrosis, but can also reflect its different development stages indirectly.
Even so, the mechanism of dust particles produced pulmonary fibrosis is unclear. One possible mechanism of lung degradation is that Si, Fe, and Al in the dust directly disrupt the signal transduction among fibroblasts, increases fibroblast proliferation (Hefflin et al., 1994; Meng et al., 2003; Wang et al., 2006). Another possible mechanism could be that the heavy metals and pathogens present in the dust cause oxidative stress in the lung (Gan and Liu, 2006). The reactive oxygen subsequently injures the lung, which gets progressively worse with increasing time (Xu and Qu, 2007). Otherwise, it could be clearly indicate that particle's acutely poisonousness causes lung inflammation by activating macrophages, which injure lung epithelial and basal cells (Feng et al., 1995; Gao et al., 2008; Gao et al., 2014). With the increase of fine dust particle's accumulated time in the respiratory system, a large number of collagen deposited in the pulmonary interstitium, eventually resulting in pulmonary fibrosis (Huang et al., 2004a, b; Wang et al., 2006; Radisky and Przybylo, 2008).
Previous studies have shown that the occurrence of pulmonary fibrosis, with the aggravation of fibrosis, blood gas analysis will be a significant change. However, during this study, the blood gas analyses of the all three groups showed no significant differences. Possible reasons for this include: a) insufficient exposure time to the dust, such that the damage to the lung tissue had not yet reached a level that would change blood gas analysis, b) the latent effect of dust on lung tissue is not fully reflected, and c) the sample size was not large enough to satisfy the statistical processing. Future studies should extend the testing time and increase the sample size in order to further investigate the impact of dust storms on the blood gas analyses of rats.
5 ConclusionExposure to dust concentrations over time results in detectable changes in lung HRCT images, but showed no significant effect on blood gas analysis. Damage to lung tissues was positively correlated with the exposure times. Exposure under sand-dust storms with certain concentrations in several days can lead to pulmonary fibrosis of rats. The mechanism for changes in lungs may be related to oxidative stress, cytotoxicity, and fibroblasts signal transduction. The studies may provide some evidencefor clinical diagnosis of lung disease caused by sand-dust storms.
Acknowledgments:This study was supported by the National Natural Science Foundation of China (41161019,41461020). We would like to acknowledge the two anonymous referees and the administrative editor LiangYing Sun for their constructive comments and suggestions that helped to improve the manuscript.
| Feng B, Xu GG, Zhang XS, et al, 1995. The studies of toxicity and potential lung fibrosis of desert dust. Journal of Health Toxi-cology, 9(4): 223–225. |
| Fennerty A, Hunter AM, Smith AP, et al, 1983. Silicosis in a Pakistani farmer. British Medical Journal, 287: 648–649. |
| Gan HY, Liu XJ, 2006. Expression of transforming growth fac-tor-ß1 and the type Ⅰ and type Ⅱ receptor for TGF-ß1 in lung of bleomycin-induced pulmonary fibrosis. International Journal of Respiration, 26(11): 860–861. doi: 10.3760/cma.j.issn.1673-436X.2006.11.017 |
| Gao JX, Wang ZQ, Wang SG, et al, 2008. Toxic effect of desert dust of northwest areas of China on alveolar macrophages of rats. Journal of Environment and Health, 25(12): 1056–1058. |
| Gao YR, Zhang YX, Gao B, et al, 2014. Effects of desert dust with different dispersity on lung injury and secretion of inflamma-tory cytokines in macrophages of rats. Journal of Environment and Health, 31(1): 33–36. |
| Hefflin BJ, Jalaludin B, McClure E, et al, 1994. Surveillance for dust storms and respiratory diseases in Washington State 1991. Archives of Environmental Health:An International Journal, 49(3): 170–174. doi: 10.1080/00039896.1994.9940378 |
| Hirsch M, Bar-Ziv J, Lehmann E, 1974. Simple siliceous pneu-moconiosis of Bedouin females in the Negev Desert. Clinical Radiology, 25(4): 507–510. doi: 10.1016/S0009-9260(74)80135-2 |
| Huang XL, Jin Y, Guo XB, 2004a. Study on the effects of PM2.5 and PM10 in sand storm dust on secretion of inflammatory factors in alveolar macrophages of rat. Journal of Environment and Health, 21(1): 38–40. doi: 10.16241/j.cnki.1001-5914.2004.01.017 |
| Huang XL, Jin Y, Guo XB, et al, 2004b. 2004b. Impact of dust storm PM2.5 and PM10 on the phagocytic function of alveolar macrophages of rat. Journal of Hygiene Research, 33(2): 154–157. doi: 10.3969/j.issn.1000-8020.2004.02.008 |
| Huang YX, Wang BJ, 2001. The relationship between respiratory system disease and the sandstorm weather in Lanzhou. Gansu Meteorology, 19(3): 41–44. doi: 10.3969/j.issn.1006-7639.2001.03.014 |
| Lei FF, Dang YM, Zhang ZS, 2015a. Pathology of effects of dust storm on rat lung tissue. Chinese Journal of Pathology, 44(3): 199–201. doi: 10.3760/cma.j.issn.0529-5807.2015.03.010 |
| Lei FF, Wang XB, Liu H, 2015b. Effect of dust aerosol exposure on lung function and lung histopathology in rats. National Medical Journal of China, 95(32): 2634–2638. doi: 10.3760/cma.j.issn.0376-2491.2015.32.015 |
| Meng ZQ, Hu M, Guo XB, 2003. Effects of dust storms on human health. China Journal of Public Health, 19(4): 471–472. doi: 10.3321/j.issn:1001-0580.2003.04.057 |
| Meng ZQ, Lu B, Zhou Y, 2006. Association of dust events with daily respiratory hospitalization:a time series approach (1995-2003). Acta Scientiae Circumstantiae, 26(11): 1900–1908. doi: 10.3321/j.issn.0253-2468.2006.11.026 |
| Meng ZQ, Yang ZH, Pan JJ, 2008. Cases of non-occupational pneumoconiosis from farmers in Minqin county, a desert area in Northwest China. Asian Journal of Ecotoxicology, 3(4): 337–342. |
| Radisky DC, Przybylo JA, 2008. Matrix metalloproteinase-induced fibrosis and malignancy in breast and lung. Proceedings of the American Thoracic Society, 5(3): 316–322. doi: 10.1513/pats.200711-166DR |
| Wang FF, Zheng CJ, Guo XB, 2006. Effect of PM2. 5 collected during the dust and non-dust periods on the viability and gap junctional intercellular communication in human lung fibro-blasts. Journal of Hygiene Research, 35(1): 26–30. doi: 10.3969/j.issn.1000-8020.2006.01.009 |
| Wang SG, Dong GR, Chen HZ, 2000. Advances in studying sand-dust storms of China. Journal of Desert Research, 20(4): 349–356. doi: 10.3321/j.issn:1000-694X.2000.04.002 |
| Wang ZQ, Wang SG, LianSQ, et al, 2010. Review on research status of desert pneumoconiosis. Journal of Desert Research, 30(1): 40–45. |
| Xu P, Qu JM, 2007. Oxidative stress in lungs and pharmacological intervention with antioxidants. International Journal of Respi-ration, 27(7): 540–543. doi: 10.3760/cma.j.issn.1673-436X.2007.07.016 |
 2016, 8
2016, 8


