Article Information
- Bing Liu Yu, Rong Li Xin, Shan Zhang Zhi, Jun Li Xiao, Wang Jin . 2016.
- The adaptive significance of differences of root morphology, anatomy and physiology from three ecotypes of reed (Phragmites communis Trin.)
- Sciences in Cold and Arid Regions, 8(3): 196-204
- http://dx.doi.org/10.3724/SP.J.1226.2016.00196
Article History
- Received: September 21, 2015
- Accepted: May 16, 2016
2. Key Laboratory of Stress Physiology and Ecology in Cold and Arid Regions of Gansu Province, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
Plants have evolved intricate strategies to withstand environmental stress. Although it is well-known that plants change their primary and secondary metabolism in leaves to resist and tolerate aboveground environmental stress, there is little awareness of the role of roots in these processes. This is surprising given that plant roots are responsible for the synthesis of plant toxins, play an active role in environmental sensing and defense signaling, and serve as dynamic storage organs to allow regrowth (Erb et al., 2009). Manschadi et al. (2008) studied the genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat. Monshausen and Gilroy (2009) reviewed how roots perceive physical cues such as soil water status and mechanical properties and translate them into physiological signals to redirect organ growth and modulate root system architecture. Vadez (2014) focused on how root traits might provide the most promising avenues for drought adaptation. Thus, the mechanism of signal transduction between roots and aboveground organs are providing important models in understanding the function of stress-tolerance in plants. What remains to be determined is the precise identity of most sensors used by roots to sample the soil environment. Hence, studying roots is essential for a solid understanding of resistance and tolerance to stress.
Phragmites communis Trin. (reed)is a hydrophytic species found in typical habitats of fresh and brackish swamps, riverbanks, and lakesides. In northwestern China, P. communis are widely distributed in drought or/and high salt conditions including Tengger and Badain Jaran deserts, Hexi Corridor, Qaidam and Tarim basins. They can adapt to these adverse terrestrial habitats through the evolution of genetic differences, which provide resistance to drought, salinity, and low temperatures (Haslam, 1970; 1975; Matoh et al., 1988). P. communis stable variations of morphology, physiology and genetic characteristics in response to drought and salinity over long-term evolution have produced different terrestrial ecotypes (Chen and Zhang, 1991; Zhu et al., 2003; Chen et al., 2006; Chen et al., 2007). Also, the micromorphology of leaf surface, anatomy, chloroplast ultrastructure and physio-chemical characteristics of P. communis Trin. have been contrasted among these ecotypes such as dune reed (DR), Gobi salt reed (GSR)and swamp reed (SR) (Liu et al., 2012). The specific characters of glands, waxes, xylem, phloem, sclerenchyma cells, calcium oxalate crystals, chloroplast morphology and structure, and antioxidant ability could contribute to the high resistance to salinity or drought by influencing the adaptive responses at the whole-leaf level between different ecotypes of reeds. Compared with adaptive characteristics at the leaf level, there is no evidence in response of morphology and anatomy to environmental conditions at the root level in different ecotypes of reeds. The main objective of the present study is to determine the adaptive characteristics of root morphology, cross-section anatomy and physiological features of three different reed ecotypes in the long time adaptation to their natural habitats with severe stress factors such as salinity (in GSR)or drought (in DR).
2 Material and methods 2.1 Materials selected and treatmentThe sampling sites for P. communis are located at thesoutheast edge of Tengger Desert in China, in three terrestrial ecotypes referred to as DR (dune reed), GSR (Gobi salt reed)and SR (swamp reed) (Figure 1). The introduction of the study area, morphological characteristics of plant species and soil properties (Table 1)are described in detail in Liu et al. (2012) . Top soil pH, EC, total salt and exchangeable Na in the GSR site were significantly higher than those in DR and SR sites (Table 1). Root sample analysis was performed at the same time with leaf (Liu et al., 2012). Root fragment samples after 2 cm from the root tip were harvested from the three reed ecotypes, washed cleanly in distilled water and then fixed immediately in a phosphate buffer (pH 7.2) containing 3% glutaraldehyde for 24 hours. For analyzing physiological characteristics, roots were washed and then frozen in liquid N2.

|
| Figure 1 Sampling sites and natural habitats of three reed ecotypes.Pictures adopted from Liu et al. (2012) |
| Soil properties | DR | GSR | SR |
| RWC (%) | 3.10 ± 0.13 a | Saturated b | Saturated b |
| pH | 7.44 ± 0.31 a | 10.20 ± 0.43 b | 6.80± 0.35 a |
| EC (m/s) | 0.11 ± 0.04 a | 7.99 ± 0.34 b | 0.15 ± 0.06 a |
| Total salt (g/kg) | 0.40 ± 0.07 a | 18.60 ± 0.94 b | 0.73 ± 0.14 a |
| Organic C (g/kg) | 0.43 ± 0.08 a | 15.45 ± 0.95 b | 21.53 ± 1.12 c |
| Total N (g/kg) | 0.17 ± 0.05 a | 5.29 ± 0.53 c | 2.24 ± 0.32 b |
| C:N ratio | 2.53 ± 0.85 a | 2.92 ± 0.91 a | 9.61 ± 1.05 b |
| Exchangeable K+ (cmol/kg) | 0.36 ± 0.05 a | 1.40 ± 0.21 b | 0.43 ± 0.06 a |
| Exchangeable Na+ (cmol/kg) | 0.09 ± 0.02 a | 6.90 ± 0.74 b | 0.25 ± 0.03 a |
| Values followed by the same letter within a column are not significantly different (P < 0.05) . | |||
Root length density (RLD, root length per unit soil volume)at 0-10, 10-20, and 20-30 cm soil depths was calculated based on root length and soil volume data using a root box method (Huang and Fry, 1998).
2.3 Analysis of morphological, anatomical and physiological characteristics of thin rootsSample preparation, observation with light microscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM)and measurement of physiological characteristics were performed with the root analysis, with details reported in the methods of Liu et al. (2012) .
2.4 Statistical analysisResults are expressed as means ± standard errors. SPSS v16.1J software was used for statistical analysis. To assess the statistical significance of the treatment differences, a one-way analysis of variance (ANOVA)was performed, followed by Duncan’s multiple range test at P < 0.05.
3 Results 3.1 Root length densityRoot length density (RLD)did not differ between DR and GSR (Table 2). However, RLD of SR was higher than that of DR and GSR. Roots were lacking in GSR at 10-30 cm and DR at 20-30 cm soil depths. Also, a higher percentage of total RLD was distributed in the upper 10 cm soil depth for GSR (100%)and DR (75%)than for SR (56%).
| Ecotype | Soil depth (cm) | ||
| 0-10 | 10-20 | 20-30 | |
| DR | 0.09 a | 0.03 a | 0.00 a |
| GSR | 0.03 a | 0.00 a | 0.00 a |
| SR | 0.64 b | 0.38 b | 0.12 b |
| Values followed by the same letter within a column are not significantly different (P = 0.05) . | |||
SEM micrographs (Figure 2)show that GSR and SR have trichomes in the epidermis and their epidermal cells are regular array, other than in DR without trichomes. The extent of root trichome development was very low and fewer root trichomes were present on the epidermis of thin roots of DR (Figure 2a, d). Thin roots had more and longer root trichomes in SR (Figure 2c)than in GSR (Figure 2b, e), and these trichomes were not desiccated and withered compared to GSR (Figure 2f).
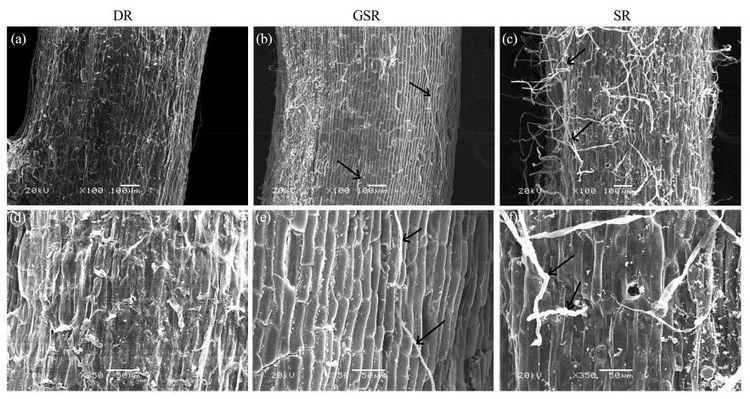
|
| Figure 2 Scanning electron micrographs showing trichome development of three reed ecotypes. (a)- (c): the whole surface view; (d)- (f): large magnification of the trichomes. Arrows indicate trichomes. (a)- (c): scale bars = 100 mm. (d)- (f): scale bars = 50 mm |
Root diameter was not significantly different among the three ecotypes, but cross-section anatomical structures of thin roots in the three ecotypes were completely different (Table 3, Figure 3). Several layers of exodermis cells surrounded by epidermal cells were observed in DR and GSR (Figure 3a and 3b). In DR, the epidermis and external cortex fall off from the root system (Figure 3a), and the largest proportion of xylem and stele in the cross section was observed compared to GSR and SR (Figure 4g, Table 3). In GSR, the largest proportion of cortical parenchyma and lysigenous aerenchyma caused by cell collapse was found (Figure 3b). The greatest proportion of cortex parenchyma with evenly distributed cells existed in SR (Figure 3c, f). The thick-walled cells between endodermis and exodermis formed sclerenchyma tissue in GSR (Figure 4e, h). This external cortex that has a lignin and/or suberin lamella covered by a thick cellulose wall may form a mechanical protection controlling water and solute flow in the roots. A sclerenchyma ring delimited a central cylinder with sclerenchyma stele is present in GSR (Figure 4k), along with scattered cortical parenchyma surrounding the stele (Figure 3e, h).
| Characteristics | DR | GSR | SR |
| Thin root diameter (mm) | 0.97 ± 0.01 a | 0.93 ± 0.01 a | 0.83 ± 0.03 a |
| Layers of epidermis cell | 1 or fall off | 1 or fall off | 1 |
| Layers of exodermis cell | 4 ± 1 b | 5 ± 1 c | 3 ± 1 a |
| Cortex parenchyma thickness (mm) | 0.12 ± 0.01 a | 0.255 ± 0.01 b | 0.37 ± 0.02 c |
| Stele diameter (mm) | 0.48 ± 0.01 b | 0.21 ± 0.01 a | 0.18 ± 0.01 a |
| Xylem proportion in stele (%) | 62.68 ± 2.55 c | 0.143 ± 0.28 a | 30.35 ± 0.94 b |
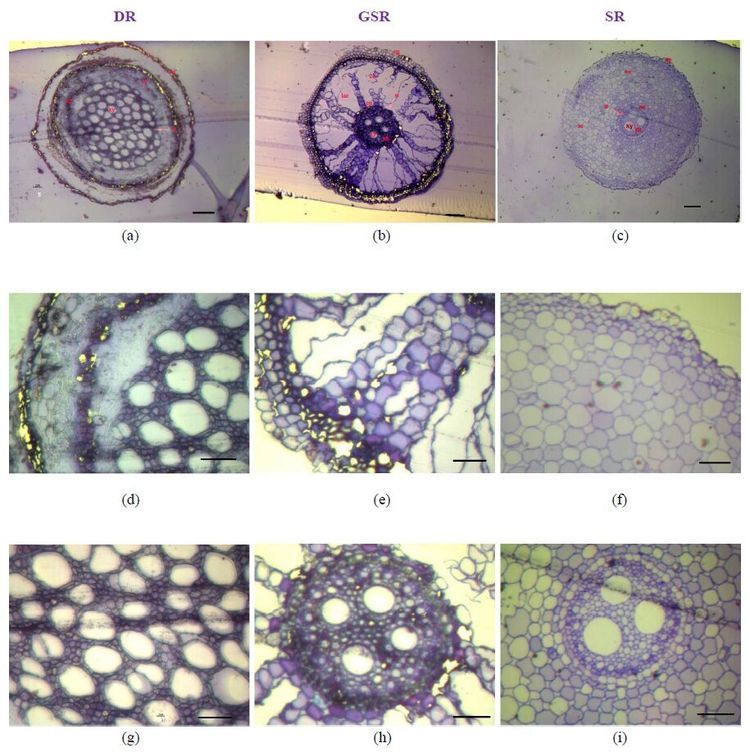
|
| Figure 3 ep, epidermis; en, endodermis; co, cortex; ae, aerenchyma; lae, lysigenous aerenchyma; st, stele (Arrows indicated); xy, xylem; ph, phloem. (a)- (c): magnification 10×, scale bars = 100 mm; (d)- (i): magnification 40×, scale bars = 50 mm (a)- (c): Microphotographs showing the whole view of the root cross section (a): DR with dropped epidermis and greater proportion of stele; (b): GSR with large proportion of cortex parenchyma and air gaps (lysigenous aerenchyma)caused by cell collapse; (c): SR with greater proportion of cortex and evenly distributed cells in the parenchyma (d)- (f): Microphotographs with details of the root cross section showing the epidermis, external cortex and cortex parenchyma of root system (g)- (i): Microphotographs with details of stele and xylem in root |
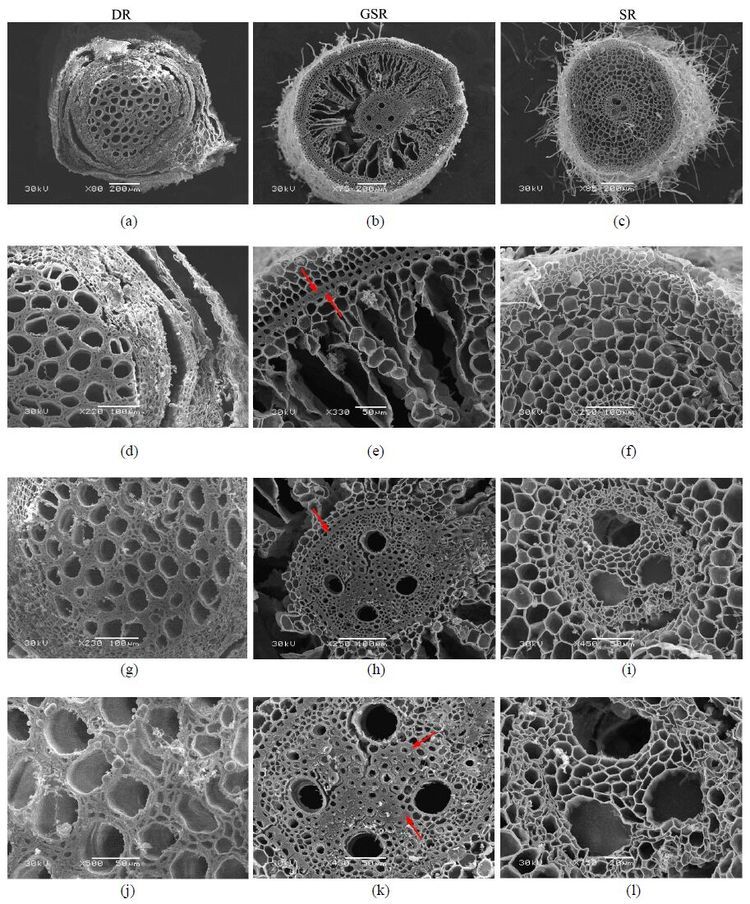
|
| Figure 4 Scanning electron micrographs showing root cross-section for three different reed ecotypes. Arrows indicate sclerenchyma tissue (scl)in the exodermis, endodermis and stele. (a)- (c): scale bars = 200 mm. (d), (f)- (h): scale bars = 100 mm. (e), (i)- (k): scale bar = 50 mm. (l): scale bar = 20 mm (a)- (c): Microphotographs showing the whole view of the root cross section (d)- (f): Microphotographs with details of the root cross section showing the epidermis, exodermis and cortical parenchyma of root system (g)- (i): Microphotographs with stele details of root system (j)- (l): Microphotographs with details of the xylem and phloem cells |
Most of the exodermal cells were irregularly shaped except epidermal cells in DR. This is in accordance with the fact that epidermis and external cortex fall off from the root system due to shortage of water absorption from the soil (Figure 5a). In GSR and SR (Figure 5b, c), the epidermal and exodermal cells are orderly arranged in spherical or square pattern, even with thick cell walls in GSR. In the exodermal cells, suberin lamella is present in DR (Figure 5d)and a sclerenchyma ring is in GSR (Figure 5e), while no such structures was observed in SR (Figure 5f).
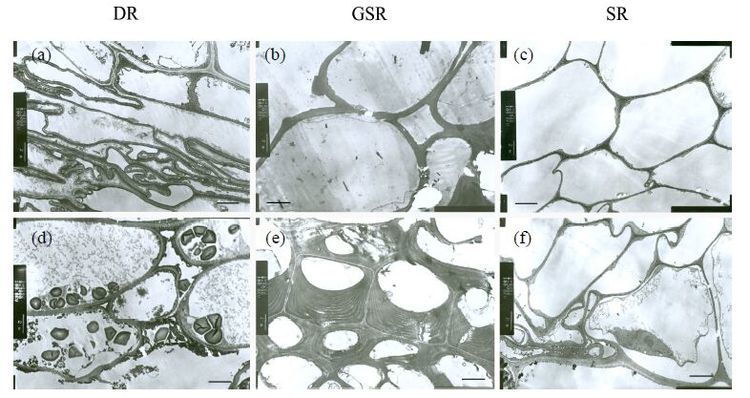
|
| Figure 5 Transmission electron micrographs of epidermal cells (a-c)and external cortex cells (d-f)of thin roots in three different ecotypes of reed. Scale bars = 2 mm |
DR and GSR had significantly higher concentrations of NO, H2O2 and lipid peroxidation than SR (Table 4). These results were consistent with antioxidative enzymes. T-AOC and CAT was significantly higher in GSR and SOD activities were significantly higher in both DR and GSR. GR activity did not significantly vary among the different ecotypes. Na+/K+-ATPase and Ca2+/Mg2+-ATPase activities were significantly higher in GSR.
| Parameter | DR | GSR | SR |
| MDA content (µmol/g DW) | 6.32 ± 0.122 b | 9.21 ± 0.232 b | 2.24 ± 0.087 a |
| H2O2 content (nmol/g DW) | 2.25 ± 0.049 b | 3.02 ± 0.063 c | 0.72 ± 0.026 a |
| NO content (nmol/g DW) | 3.22 ± 0.267 b | 5.68 ± 0.423 c | 1.23 ± 0.124 a |
| T-AOC (U/mg protein) | 126.7 ± 9.38 ab | 174.2 ± 10.42 b | 84.3 ± 9.22 a |
| SOD activity (U/mg protein) | 5.31 ± 0.166 b | 6.37 ± 0.187 b | 2.45 ± 0.165 a |
| CAT activity (U/mg protein) | 22.4 ± 2.25 a | 33.5 ± 2.32 b | 19.8 ± 2.03 a |
| GR activity (U/mg protein) | 3.22 ± 0.182 a | 3.68 ± 0.196 a | 3.54 ± 0.179 a |
| Na+/K+-ATPase activity (U/mg protein) | 0.548 ± 0.0463 a | 0.963 ± 0.0548 b | 0.498 ± 0.0385 a |
| Ca2+/Mg2+-ATPase activity (U/mg protein) | 0.825 ± 0.0954 a | 2.745 ± 0.846 b | 0.901 ± 0.0536 a |
DR and GSR in the natural habitat are subjected to drought stress, and high salinity for GSR. Plant tissue response to water stress depends on physiological properties of the cell components and the anatomic characteristics that regulate the transmission of the water stress effect to the cells. The difference in the response to water stress among mature regions and regions of tissue growth seems to be due to anatomic differences (Matsuda & Rayan, 1990).
More profuse (higher root length density, RLD)and deeper root systems are often viewed as desirable traits for drought adaptation (Kashiwagi et al., 2006; Gowda et al., 2011). While roots are potentially important for plants under drought stress, they do not contribute to drought adaptation in all stress conditions since in many cases the degree of differences in root growth among genotypes do not explain the degrees of differences in yield (Vadez, 2014). P. communis in this study is a clone plant where breeding depends on underground stems. Thus, the characters of root length density (RLD)and root morphology in DR and GSR did not show a distinct drought or salinity tolerance. However, root anatomy shows a distinct difference.
Root anatomical alterations may occur in plants under environmental stress to protect and adapt the species to the stress. These alterations are probably due to lignin or suberin deposits found in the exodermis, endoderm and cell layers adjacent to the root cortex and medulla (Baruch & Mérida, 1995)that protect against stress and cortex cell death (Sharp & Davies, 1985). Plant roots have two 'physiological sheaths' (endodermis, exodermis)that play important roles in basic root function and protection against stress (Enstone et al., 2003). Tissues exposed to environments with low water availability have generally shown reduction in cell size, increase in vascular tissue and cell wall thickness (lignification) (Levitt, 1980; Pitman et al., 1983).
From our results, the main anatomical characteristics of the three ecotypes are summarized as follows: DR with dropped epidermis and greater proportion of stele; GSR with large proportion of cortex parenchyma and air gaps (lysigenous aerenchyma)caused by cell collapse; SR with greater proportion of cortex and evenly distributed cells in the parenchyma. Stress is known to induce the formation of an exodermis and to accelerate the development of both the exodermis and endodermis (Enstone et al., 2003). Parenchyma cell loss in DR may also be a strategy to reduce water efflux from roots in drying soil. Large proportion of stele and xylem areas and suberin lamella presented in DR may be related to water absorption and storage in an arid desert environment. Thickened and histochemically differentiated cell walls of the exodermis and endodermis are characteristic of DR and GSR roots. The endodermis and exodermis are potentially important for plant resistance to salinity (Enstone et al., 2003). The external and internal cortex that has a sclerenchyma ring may form a mechanical protection controlling water and solute flow in the roots of GSR. This compact external cortex may also have the function of protecting the cortex cells from collapse due to the hydrostatic pressure in the submerged roots and may also protect against desiccation and death of the root cortex cells exposed to water shortage. These tissues appear to function in the absorption and transport of water and solutes (Tomlinson, 1969; Barnabas, 1994), and in the mechanical processes of anchorage and plant support (Barnabas, 1991, 1994). GSR adapted to flooded and salinity environments and therefore presented the external cortex with a thick cell layer showing that this thickening is part of survival strategies under adverse conditions of excess water. Air spaces in cortical tissue could interrupt radial water movement in roots and reduce root/soil contact because of root shrinkage away from the soil, thus limiting water uptake in drying soils (Huck et al., 1970). In very dry soil, however, when soil water potential drops below root water potential, air space formation prevents water loss from plants to the dry soil in desert succulents (Nobel and Huang, 1992). The presence of aerenchymas in GSR helps the plants under conditions of excess water in the soils to maintain aerobic respiration by maintaining O2 diffusion (Kawase, 1981). According to Huang & Fry (1998) there is a collapse of the cortex cells that gives rise to the aerenchymas that in soils with severe water shortage may help to prevent water loss from the plants.
Physio-chemical parameters were higher in roots of GSR, and then in DR, which in accordance with the leaves (Liu et al., 2012), including contents of MDA, H2O2 and NO, a higher level of antioxidant activities, and higher activities of Na+/K+ and Ca2+/Mg2+ ion transport. This will contribute to interpret the high resistance to salinity or drought by influencing the adapting responses at the physiological level among different ecotypes of reeds.
5 ConclusionsThe anatomical and physiological assessment of roots in three reed ecotypes shows the plants responded to their natural habitat conditions. The developed water-absorbing tissues were largely distributed in the root structure of DR and a main framework with supporting function spacing with aerenchyma was dominant in GSR. The physio-chemical parameters show that GSR had a higher level of stress tolerance than DR. These findings show that adaptation to different levels for survival under adverse stress contributed to understanding their establishment in regulated mechanism of root system in different reed ecotypes. However, it is pointed out that complementary studies in different reed ecotypes under different natural conditions that assess the establishment of roots, associated with regulation of root hydraulic conductivity such as water and nutrient availability in the soil, aquaporin and genetic variation, are essential to approach the question of regulating plant water use. Thus, understanding the water use strategy of different reed ecotypes may help to resolve the problem of re-vegetation in arid desert conditions and salt lakes.
Acknowledgments:This study was financially supported by the State Key Development Program for Basic Research of China (973 Program, Grant No. 2013CB429904) and the National Natural Science Foundation of China (Grant No. 91125029) .
 2016, 8
2016, 8


