Article Information
- XiaMei Yao, Jing Ma, Jing Ji, Chun Ou, WenQiang Gao. 2016.
- Effect of exogenous application of salicylic acid on the drought stress responses of Gardenia jasminoides
- Sciences in Cold and Arid Regions, 8(1): 54-64
- http://dx.doi.org/10.3724/SP.J.1226.2016.00054
Article History
- Received: August 7, 2015
- Accepted: October 6, 2015
2. State Key Laboratory of Tree Genetics and Breeding, Research Institute of Forestry, Chinese Academy of Forestry Sciences, Beijing 100091, China
1 Introduction
Plants are often exposed to various environmental stresses under natural conditions. Drought stress is one of the major environmental factors limiting the distribution and growth of l and plants. The harmful effects of this stress are ranked first among numerous abiotic stresses. More than a third of the world's l and area is located in arid and semi-arid regions. Moreover,in other regions,different degrees of drought also occur in the plant growing season,the essence of which is plant water shortage,namely,drought stress. How to make plants grow normally in arid environments,as well as ensure their yield and quality,has become a long-st and ing need for researchers trying to solve this problem. Adding exogenous substances and regulating plant antioxidant components are possible solutions to abiotic stresses on plant damage(Hashempour et al., 2014).
Salicylic acid(SA)is a small antioxidant molecule that is soluble in water and plays a crucial regulatory function,which is involved in a number of physiological processes in plant growth and development. As one of the most commonly used exogenous hormone substances,SA has been discovered to act as an important role in improving plant resistance to biotic and abiotic stresses since the 1960s(Singh and Gautam, 2013). Moreover,SA is also an important signaling molecule in the induction of systemic acquired resistance. Several lines of evidence prove that exogenous application of SA can enhance hydrogen peroxide(H2O2)content in plants exposed to different environmental stress, and induce the expression of gene-related antioxidant enzymes. However,improvement of antioxidant enzyme(e.g.,superoxide dismutase(SOD),peroxidase(POD), and catalase(CAT))activity in plants can efficiently reduce damage caused by reactive oxygen species(ROS)accumulation,which ultimately induces the expression of several genes involved in stress reaction. Simultaneously,SA also lowers membrane lipid peroxidation product malondialdehyde(MDA)accumulation and leaf membrane permeability,as well as an increase in ATP content in plants to provide sufficient energy for normal metabolism of various substances,which can improve plant resistance to high salt,low temperature,heavy metal, and other environmental stress. To date,however,several studies have reported the regulatory role of exogenous substances,in which treatment effect depends on application methods,timing,exogenous substance concentration,stress environment,as well as the plant species tested(Idrees et al., 2012).
Gardenia(Gardenia jasminoides J. Ellis)is a high-quality greening shrub because of its shade tolerance and anti-gas capability. It iswidely distributed in various regions of China. Furthermore,this shrub was demonstrated in previous research to belong to the first batch of medicinal and edible species issued by the Ministry of Health. Moreover,its fruit is a traditional Chinese medicine that is internationally popular as natural pigments and food additives because it contains a natural yellow pigment. Current research on G. jasminoides mostly focused on pharmacological effects(Song et al., 2014),cultivation growth(Lykas et al., 2006), and process value(Jarvis et al., 2014). However,research associated with the physiological recovery of G. jasminoides seedlings under drought stress has yet to bereported. Drought stress is a critical abiotic stress factor that affects the metabolism and yield of G. jasminoides. Therefore,discovering methods to improve drought resistance and analyze its internal mechanism for regulating the growth and development of G. jasminoides based on its economic and ornamental value is of vital significance. In addition,current considerable progress achieved in research concerning the role of SA in drought resistance mainly focused on herbs,whereas easing responses to drought stress of shrubs,such as G. jasminoides,is rarely addressed. Polyethylene glycol(PEG)-simulated drought stress has been widely used to investigate plant adaptive mechanisms(Patade et al., 2009). In present study,to clarify the role of two types of exogenous substances(SA)in the plant response to drought stress,we used PEG6000 to treat G. jasminoides seedlings and then comparatively analyzed their performances.
2 Methods 2.1 Plant growth and treatmentIn this study,1.5-year-old G. jasminoides seedlings of uniform size were obtained from Fuyang Nursery Base in Anhui,China. Initially,after sterilization with 0.5% potassium permanganate for 15 min followed by repeated thorough washing with distilled water,these uniform-sized seedlings were transferred into plastic containers(500 mL,three plants per container)filled with perlite, and watered with 1/2 Hoagl and nutrient solution at pH 6.5 for 14 days. Seedlingswith identical growth,after resuming and growing normally,were transferred to plastic containers(2,000 mL,12 plants per container)with fresh nutrient solution and then subjected to different treatments for 14 days. Moderate drought stress was simulated using 15%(−0.388 MPa)PEG6000 treatment(Michel and Kaufmann, 1973).These seedlings were grown in a growth chamber with a 12 h/12 h light/dark cycle,day/night temperature of 25 ℃/18 ℃,light intensity of 2,000 lx at the leaf level, and relative humidity of 65%±5%. The seedlings were then grouped into 10 experimental test sets: CK(1/2 Hoagl and nutrient solution),PEG(moderate drought stress,15% PEG6000-treated nutrient solutions),PEG+SA0.5(0.5 mmol/L SA + 15% PEG6000),PEG + SA1(1 mmol/L SA + 15% PEG6000),PEG+SA2(2 mmol/L SA + 15% PEG6000),PEG + SA4(4 mmol/L SA + 15% PEG6000). SA solutions were respectively sprayed on leaves of G. jasminoides grown in 15% PEG6000-treated nutrient solutions based on different treatments. Spraying was carried out in the morning and evening every day for 14 days.
The treatments were performed in the form of a completely r and omized block design with three repetitions. Hydroponic solutions were renewed once a day during the experiment to maintain constant concentrations. For the determination of physiological and biochemical parameters,fresh leaf samples were harvested,promptly frozen in liquid nitrogen, and stored at −70 ℃.
2.2 Determination of plant growth and relative water content(RWC)After 14 days of growth under the aforementioned conditions,the leaves and roots of plants were carefully separated,washed with 0.5%(w/v)potassium permanganate, and then washed thoroughly with distilled water. PEG6000-treated plants and their controls were dried in an oven at 105 ℃ for 30 min, and then at 80 ℃,until the dry weight was stabilized. The dry materials were weighed using a digital analytical balance. In addition,the height of plants and length of primary roots were measured from seedlings of each treatment set.
Fresh leaves collected from plants were promptly weighed(fresh weight),placed in water for 24 hours at 4 ℃ in the dark(turgid weight), and dried in an oven at 80 ℃ to constant mass(dry weight). RWC was calculated as 100 ×(fresh weight − dry weight)/(turgid weight − dry weight).
2.3 Determination of photosynthetic pigment content and photosynthesisThe chlorophyll content was assessed according to the method described in the study of Knudson et al.(1977). Approximately 0.2 g of fresh leaves was extracted using 95% ethanol with three replications. The absorbance of the extracted solution was measured at 665,649, and 470 nm by a spectrophotometer. Chlorophyll a,chlorophyll b, and carotenoid(car)contents were determined based on the formula and then expressed as milligram per gram fresh weight(mg/g).
Net photosynthesis(Pn),transpiration rate(Tr), and stomatal conductance(Gs)of G. jasminoides leaveswere measured using a portable photosynthesis system(LI-6400,LICOR)in three replications. The leaves tested were fully exp and ed,located in the upper part of the plant crown. Determinations were conducted between 10:00 am and 11:00 am in sunny and cloudless weather, and obtained at a photosynthetic photon flux density of 1,100 μmol/(m2·s). In addition,ambient temperature was approximately 25 ℃, and CO2 concentration was 350 μmol/mol. Based on Pn and Tr,the water use efficiency(WUE)was calculated.
2.4 Determination of hydrogen peroxide,lipid peroxidation, and electrolyte leakageThe hydrogen peroxide content was quantified in leaf extracts by adopting a previously described method by Velikova et al.(2000)with slight modifications.G. jasminoides leaf tissues were ground with liquid nitrogen,homogenized in cold 3% trichloroacetic acid(TCA), and centrifuged at 12,000×g for 20 min. After removal,the supernatant was mixed with an equal volume of 10 mM phosphate buffer(pH 7.0) and 1 M KI. Based on the absorbance read at 390 nm,the amount of H2O2 in leaves was determined using a st and ard curve.
The MDA content was estimated by thiobarbituric acid(TBA)according to Heath and Packer(1968)as a product of lipid peroxidation. Fresh leaf samples(0.2 g)were ground homogeneously with a mortar and pestle in 20% TCA. The supernatant was then centrifuged at 10,000×g for 15 min,mixed with 0.5% TBA, and boiled at 95 ℃ in a water bath for 30 min. After rapid cooling in an ice bath,the mixture was centrifuged again. The absorbance of the supernatant was recorded at 532 nm,whereas the value of non-specific absorbance at 600 nm was subtracted. The MDA content was calculated using an extinction coefficient of 155 mM−1·cm−1.
Electrolyte leakage was estimated according to the method of Sinhababu and Kar(2003). Five fresh leaf discs of uniform size were excised and rinsed with deionized water,slightly dried on filter paper,placed in tubes containing deionized water, and shaken at room temperature for 24 hours. The electrical conductivity of the solution obtained(EC1)was measured using a conductivity meter(DDS-11A). The tubes containing the samples were heated in boiling water at 100 ℃ for 15 min to disrupt the tissues completely and then cooled. The conductivity of the solution with killed tissues(EC2)was measured again. Based on the above EC1 and EC2,the relative electric conductivity of the extract was calculated.
2.5 Determination of antioxidant enzymatic activitiesAntioxidant enzymatic activities were determined by essentially following the method described by Dhindsa et al.(1981). SOD activity was assayed by its ability to inhibit the photochemical reduction of Nitrotetrazolium Blue chloride(NBT)at 560 nm. One unit of SOD was defined as the amount of enzyme required to inhibit the reduction of NBT by 50% at 25 ℃. POD activity was assayed in the reaction solution containing 50 mM phosphate buffer(pH 7.8),25 mM guaiacol,20 mM H2O2, and the enzyme extract by measuring the changes in absorbance of the reaction solution at 470 nm. CAT activity was assessed by measuring the initial rate of decomposition of H2O2 at 240 nm. The reaction mixture consisted of 50 mM potassium phosphate buffer(pH 7.0)containing 0.1 mM EDTA,20 mM H2O2, and the enzyme extract.
2.6 Proline determinationThe proline content in leaves was quantified according to the method of Bates et al.(1973). Fresh samples were ground in 3% aqueous sulfosalicylic acid. The extract(2 mL)was combined with 2 mL of glacial acetic acid and 2 mL of 2.5% acidic ninhydrin. After heating at 100 ℃ for 30 min and cooled,the liquid was added to 4 mL of toluene. The supernatant was centrifuged, and its absorbance was read at 520 nm. The proline concentration was obtained based on the st and ard curve.
2.7 Statistical analysisAll data obtained from the experiments are expressed as the mean ± st and ard deviation of three replications. All statistical analyses were performed with SPSS statistical package version 16.0. One-way ANOVA was conducted to compare the means. Differences between treatments were separated by the least significant difference test at 0.05 probability level.
3 Results 3.1 Plant growth and RWCAs presented in Table 1,the aboveground and underground dry mass,seedling height, and root length in G. jasminoides plants significantly decreased to 14.03 g,5.35 g,23.13 cm, and 10.53 cm,respectively,upon exposure to 15% PEG. However,this reduction in aboveground and underground dry mass was reversed by combination treatment,in which plants were subjected to PEG combined with SA. With increasing SA addition,dry mass,seedling height, and root length increased first and then decreased,achieving the best effect at 1 mmol/L SA. Similarly,the RWC of leaves also declined under PEG stress. Exogenous application of SA resulted in an increase in the RWC of stressed plants,but these increases were considerably higher than those of plants exposed to PEG alone. The RWC of PEG-stressed plants decreased to 29.56%. The RWC of plants treated with 1 mmol/L SA increased to 81.21%,which was 2.75 times more than those of stressed plants.
| Treatment | Seedling height (cm) | Root length (cm) | Aboveground dry mass (g) | Underground dry mass (g) | Relative water content (%) |
| CK | 24.75±0.29a | 11.25±0.11a | 16.86±0.33a | 6.35±0.21ab | 87.80±2.97a |
| PEG | 23.13±0.13d | 10.53±0.08d | 14.03±0.24d | 5.35±0.19d | 29.56±0.91e |
| PEG+SA0.5 | 24.56±0.35ab | 11.20±0.11a | 16.43±0.31ab | 6.21±0.21ab | 70.92±4.94b |
| PEG+SA1 | 24.79±0.33a | 11.34±0.09a | 16.94±0.34a | 6.60±0.26a | 81.21±5.49ab |
| PEG+SA2 | 24.04±0.22b | 10.98±0.12b | 16.01±0.27b | 6.12±0.14b | 59.44±4.25c |
| PEG+SA4 | 23.47±0.15c | 10.62±0.12c | 15.11±0.25c | 5.72±0.12c | 32.92±3.43d |
| Note: values represent mean ± standard deviation (n = 3). Means in each column followed by different lower-case letters are significantly different (P ≤0.05) by Duncan's multiple-range test. | |||||
Variations in the levels of photosynthetic pigments in G. jasminoides were assessed under different treatments(Figure 1). The contents of photosynthetic pigments(total chlorophyll,chlorophyll a,chlorophyll b, and carotenoids)differed among different treatments. After 14 days of PEG treatment,the photosynthetic pigment content significantly decreased compared with that of the controls. With elevated doses of additional SA,the photosynthetic pigment contents were found to increase first and decrease forG. jasminoides with PEG stress,reaching the maximum at 1 mmol/L SA. PEG+SA1 significantly improved the contents of total chlorophyll,chlorophyll a,chlorophyll b, and carotenoid by 25.12%,23.65%,30.36%, and 36.60%,respectively, and these values were higher than those of PEG treatment alone at the 14th day but lower than those of control seedlings,which were higher than those on the 7th day.
 |
| Figure 1 Effects of salicylic acid (SA) on photosynthetic pigments(total chlorophyll, chlorophyll a, b and carotenoids content) of Gardenia jasminoides under PEG-simulated drought stress. Values represent mean ± standard deviation (n = 3). Means followed by different lower-case letters are significantly different (P ≤0.05) by Duncan's multiple-range test |
To evaluate the effects of SA on the photosynthesis of G. jasminoides,the net photosynthetic rate(Pn),transpiration rate(Tr),stomatal conductance(Gs), and WUE were measured, and the results are presented in Figure 2. In 15% PEG-treated seedlings,Pn,Tr,Gs, and WUE markedly decreased by 69.82%,60.95%,59.67%, and 22.67% at the 7th day, and by 73.94%,64.75%,70.54%, and 26.02% at the 14th day,respectively,in comparison with the controls. In the presence of SA,changes were induced in Pn,Tr,Gs, and WUE of PEG-stressed plants. The addition of 0.5-1 mmol/L SA enhanced Pn,Tr,Gs, and WUE of PEG-stressed plants up to the 14th day.
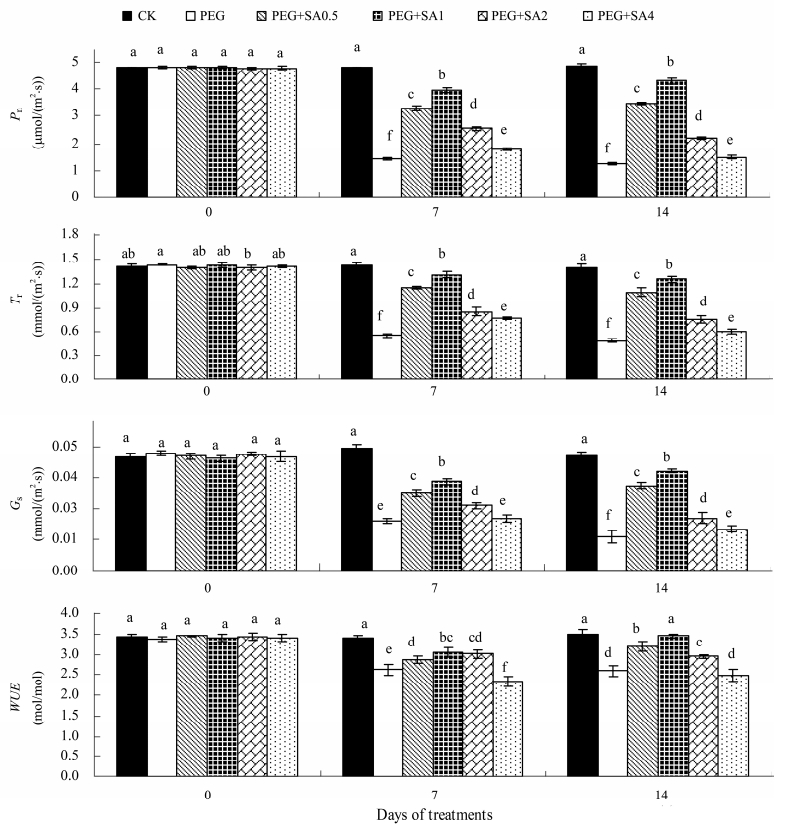 |
| Figure 2 Effects of salicylic acid (SA) on net photosynthetic rates (Pn), transpiration rate (Tr), stomatal conductance (Gs) and water use efficiency (WUE) of Gardenia jasminoides under PEG-simulated drought stress. Values represent mean ± standard error (n = 3). Means followed by different lower-case letters are significantly different (P ≤0.05) by Duncan's multiple-range test |
To assess oxidative stress generated by PEG stress,the MDA content was determined as an indicator of lipid peroxidation. The MDA content in the leaves increased significantly by 57.40% and 78.06% at the 7th and 14th day,respectively,in response to 15% PEG treatment alone(Figure 3). In the presence of PEG,application of SA resulted in different effects on the MDA content. The addition of 0.5 and 1 mmol/L SA rapidly reduced MDA accumulation in leaves on the 7th day of PEG stress by 21.29% and 24.08%,respectively,compared with PEG treatment alone,maintaining an upward trend until the final stage(the 14th day)of treatments. Conversely,no significant change was indicated between 4 mmol/L SA and PEG stress alone on the 7th day.
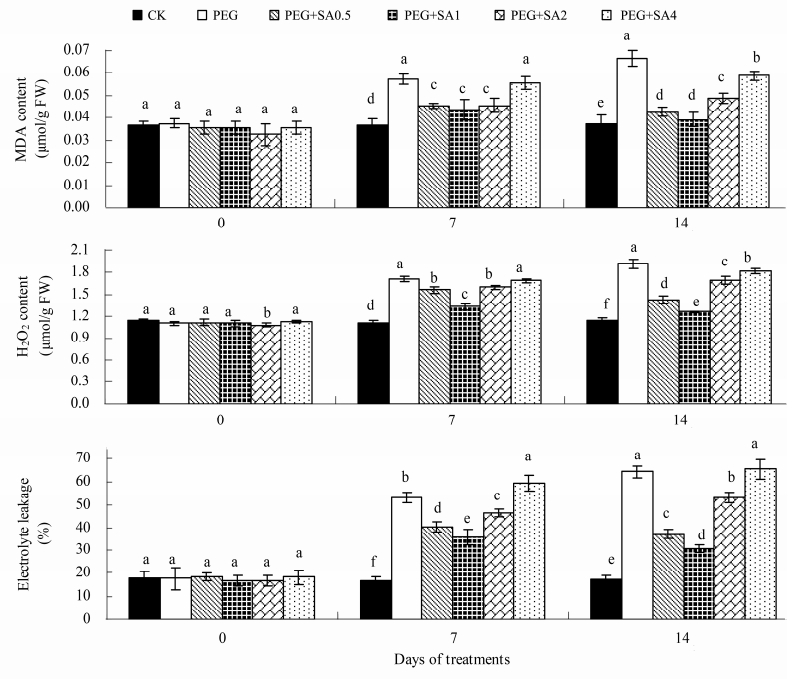 |
| Figure 3 Effects of salicylic acid (SA) on MDA, H2O2 content and electrolyte leakage of Gardenia jasminoides under PEG-simulated drought stress. Values represent mean ± standard deviation (n = 3). Means followed by different lower-case letters are significantly different (P ≤0.05) by Duncan's multiple-range test |
Under 15% PEG stress,the H2O2 content and electrolyte leakage in leaves were found to increase by 67.54% and 264.11%,respectively,at the 14th day in comparison with the respective controls. Meanwhile,by the end of treatment and upon SA application,the H2O2 content was significantly reduced by 25.17% and 34.10% in PEG+SA0.5- and PEG+SA1-treated seedlings,respectively(Figure 3),compared with that in seedlings treated with PEG alone. SA also induced a substantial change in electrolyte leakage in 15% PEG-treated seedlings,which is presented in Figure 3. Compared with PEG treatment alone,electrolyte leakage was reduced by 51.89% after 14 days of exposure to PEG+SA1. By contrast,the values of electrolyte leakage were not significantly different among PEG+SA4 and PEG treatment alone at the 14th day.
3.4 Antioxidative activity and proline contentFigure 4 shows that 15% PEG resulted in decreases by 27.86%,20.94%, and 25.24%,respectively,in the activities of SOD,POD, and CAT in the leaves of G. jasminoides on day 14 with respect to the controls. Application of SA induced variations in these three enzymes to varying degrees in conjunction with PEG stress,thereby manifesting an initial upward and then downward trend with increasing concentration. After G. jasminoides was completely sprayed with SA,SOD and POD activities of plants at 1 mmol/L SA significantly increased by 46.01% and 28.34%,respectively,compared with PEG treatment,reaching their respective highest level(289.99 and 2,714.45 U/g)at day 14 among the tested SA treatments,whereas the CAT content peaked under PEG+SA0.5 treatment at 174.75 U/g.
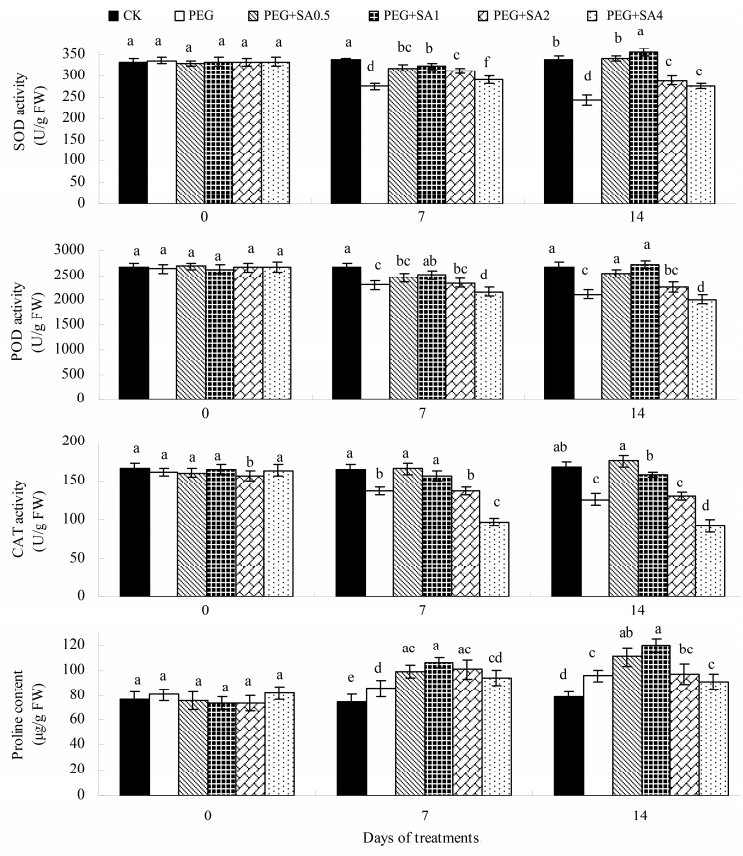 |
| Figure 4 Effects of salicylic acid (SA) on superoxide (SOD), peroxidase (POD) and catalase (CAT) activity and proline contentof Gardenia jasminoides under PEG-simulated drought stress. Values represent mean ± standard deviation (n = 3). Means followed by different lower-case letters are significantly different (P ≤0.05) by Duncan's multiple-range test |
As presented in Figure 4,proline accumulation in the leaves of G. jasminoides seedlings was markedly enhanced to 13.64% and 21.31% after 7 and 14 days of 15% PEG stress,respectively,compared with that in the controls. Compared with PEG treatment alone,exogenous 1 mmol/L SA exerted a remarkable increase in the proline content of G. jasminoides leaves at the 14th day under PEG stress,by 25.46% and 31.01%,respectively,thereby reaching their respective peak in SA treatment groups. After 14 days of treatments,no significant difference in the increase in proline content was noted when SA were continuously added to high levels(2 and 4 mmol/L SA)together with PEG treatment.
4 DiscussionSoil water condition is considered the main limiting factor that affects plant growth, and drought stress is commonly associated with the inhibition of plant growth and development. In recent years,numerous studies have shown that plants exposed to drought stress are unable to compensate for water transpiration by root uptake of water,resulting in a water deficit in plants,decrease in cell turgor pressure, and restraint of plant cell division and growth. Simultaneously,a series of physiological and biochemical metabolic processes in plants exerts changes that ultimately affect the whole plant growth,as well as morphological and physiological characteristics(Liu et al., 2011). PEG,with its molecular weight of above 6,000,is the ideal water regulation substance to simulate soil drought(Sarunyaporn et al.,2014),which has become an important means in research on plant drought resistance. Exogenous substances(e.g.,SA,ABA, and polyamines)can effectively alleviate damage in plants caused by drought stress,such as an increment of plant tissue water content,water potential, and MDA content reduction for preventing electrolyte leakage(Aroca et al., 2008). The results of the present study further supports the adverse effect of drought stress simulated by PEG,showing a decline in plant height,root length, and both aboveground and underground dry biomass observed in G. jasminoides seedlings grown under PEG stress(Table 1). A reduction in biomass production induced by drought stress has been reported by Remy et al.(2014). Inhibition of growth in G. jasminoides plants may result from PEG-caused variation of radical metabolic processes. However,the application of SA at low concentration was observed to raise the aboveground and underground dry weights(Table 1)in PEG-stressed G. jasminoides plants. Similarly,promotion of growth using SA has also been documented in Ctenanthe setosa plants(Kadioglu et al., 2011).
Photosynthetic pigment is the carrier of leaf photosynthesis, and the chlorophyll content is an important index of plant drought resistance that is closely correlated with photosynthetic intensity,largely determining plant growth condition and leaf photosynthetic capacity. Plants responding to stressful environments,such as drought,have been documented to lead to low chlorophyll levels,thereby resulting in the retardation of plant overall growth. In this study,in comparison with PEG treatment alone,advancements in the contents of photosynthetic pigments,such as chlorophyll a and chlorophyll b,total chlorophyll, and carotenoid,were significant in G. jasminoides plants treated with the combination of 1 mmol/L SA and 15% PEG,which was similar to emerging research results demonstrated in the literature(Chen et al., 2014). This finding was possibly due to protection by exogenous substances SA for the leaf photosynthetic apparatus of G. jasminoides,accompanied with maintained antioxidant enzyme activity,reduction in the accumulation of ROS,protection of thylakoid membrane pigment protein function, and relief in membrane damage caused by stress. Given the water deficit caused by stress treatment,membrane lipid peroxidation damages the chloroplast membrane system,resulting in damage to the photosynthetic mechanism,serious decline in the photosynthetic rate,decrease in WUE, and limited seedling growth. In the present study,drought stress caused by PEG lessened the net photosynthetic rates and stomatal conductance of G. jasminoides,as presented in Figure 2. Furthermore,the decrease in stomatal conductance,which diminished the transpiration rate to reduce water loss of G. jasminoides plants,restricted CO2 absorption by the leaves. This trend was the result of the joint action of several factors,such as the decline in the photosynthetic pigment content, and rising ROS content in chloroplasts.In addition,G. jasminoides plants treated with PEG alone presented lower stomatal conductance accompanied with decreased net photosynthetic rates and WUE compared with control plants. To a certain extent,this response may be a reason why biomass of PEG-treated plants is lower than that of controls during the same growth period. Fariduddin et al.(2003)demonstrated that SA can improve net photosynthetic rates and WUE,accompanied with increasing stomatal conductance,thereby enhancing plant physiological metabolic capacity and adaptation to stress; these findings suggest that changes in stomatal conductance may be the fundamental reason for increased net photosynthetic rates. In agreement with these findings,the results of the present research indicate that 0.5-1 mmol/L SA ameliorated the adverse effect triggered by PEG on photosynthesis,transpiration rates, and stomatal conductance(Figure 2),which may be attributed to an increase in the photosynthetic pigment content.
Under normal environmental conditions,active oxygen metabolism in plants remains in dynamic equilibrium because of the regulation of enzymatic systems. Under stress conditions,this balance is broken,leading to oxidative damage of plants. Survival of plants under drought stress is triggered by the induced mechanism of the antioxidant defense system. Therefore,advancement of plant resistance is closely related to its antioxidant systems(Boogar et al., 2014). In present research,the presence of PEG significantly reduced SOD,POD, and CAT activities of G. jasminoides seedlings,which demonstrated the stress reaction of the plant protective enzyme system; this observation is in agreement with the results reported by others(Yilmaz and Parlak, 2011; He et al., 2014). The activities of antioxidant enzymes,such as SOD,POD, and CAT,subjected to drought stress,along with the lowering ability of plants against oxidative damage or the rising effect generated by ROS,induced lipid peroxidation and further damaged the membrane system. The restoration of this system was inferred to be associated with an increase in the activity of these enzymes. In addition,PEG treatment was presented to increase the H2O2 content in leaves of G. jasminoides(Figure 3),which has been found in the leaves ofArabidopsis(He et al., 2014). The content of MDA,as one of the main products of membrane lipid peroxidation,is a critical indicator that is generally used to measure the extent of membrane lipid peroxidation and plasma membrane damage(Mahmood et al., 2012). The MDA content markedly increased in present research. Based on the above variation,G. jasminoides seedlings were seriously injured. However,when SA at 0.5-1 mmol/L was added to the PEG treatment solution,SOD,POD, and CAT activities(Figure 4)increased with decreasing MDA and H2O2concentrations(Figure 3). In accordance with these results,exogenous SA treatment of perennial ryegrass plants subjected to cadmium stress has been reported to mitigate oxidative stress by increasing antioxidant enzymatic activities and diminishing the concentrations of MDA and H2O2(Singh and Gautam, 2013). An appropriate concentration of SA has been suggested to induce protein synthesis,triggering the stimulation of antioxidant production, and lower MDA and H2O2 levels,which maintain stabilization of cell membranes in plants under PEG stress. By contrast,the addition of 2-4 mmol/L SA exerted a negative effect on the protective enzyme activities of G. jasminoides plants under PEG stress,which led to the delayed removal of ROS,causing serious injury to plants. Based on these changes,higher concentrations of SA are suggested to be non-conducive to plant growth,which can be supported by reports regarding tobacco(Zhang et al., 2012). Other researchers(Shi et al., 2015)have confirmed that the RWC reflects the water retention of plant leaves. When subjected to stress,the plasma membrane undergoes varying degrees of damage,resulting in increased membrane permeability,the degree of which depends on the types of plants,time, and rate. PEG stress significantly decreased the level of RWC,accompanied with an increase in electrolyte leakage. Li et al.(2013)also reported that,except for the MDA and H2O2 contents,electrolyte leakage was a symptom of stress-caused damage and deterioration. However,0.5-1 mmol/L SA treatment effectively alleviated these variations.
Several lines of evidence have shown that the accumulation of proline,as the cytoplasmic osmotic regulator,is associated with plant resistance and can be regarded as an indicator of resistance,whereas several studies have shown that the level of proline under stress is the result of plant damage(Sucre,2011). Proline observed in this study may be related to the mechanism of osmotic adjustment. G. jasminoides seedlings were subjected to PEG stress with proline accumulation(Figure 4),which is in line with previous results obtained from others(Yilmaz and Parlak, 2011). The level of proline dramatically increased upon exposure to 15% PEG,which may be relevant to the acquisition of tolerance. Subsequently,0.5-1 mmol/L SA further enhanced proline concentration in PEG-stressed plants,reaching the peak at 1 mmol/L SA. This addition partially mitigated drought stress induced by 15% PEG,probably because the application of suitable levels of SA improved the enzyme activity involved in proline biosynthesis,promoted its synthesis, and reduced the degradation of synthesized substance. Thus,the proline content and osmotic adjustment ability of G. jasminoides were effectively improved. Chen et al.(2014)reported that 1 mmol/L SA improved the proline content in zoysia grass plants under drought stress compared with other levels of SA. By contrast,high levels of exogenous SA aggravated damage associated with PEG stress possibly by proline degradation(Figure 4).
In summary,exposure of G. jasminoides plants to PEG-simulated drought stress led to decreases in photosynthetic pigments,RWC, and photosynthetic characteristics,as well as increases in antioxidant enzyme activity,electrolyte leakage,MDA content,H2O2 content, and proline accumulation. The presence of exogenous 0.5-1 mmol/L SA could offset the negative effects of drought stress by increasing photosynthetic pigments,RWC, and photosynthetic characteristics,along with rising biomass. Compared with other SA treatments,SA at more than 2 mmol/L,respectively,produced no significant effects on G. jasminoides plants,or even severely inhibited plant growth and normal physiological activity.
Acknowledgments: This research was funded by the National Natural Science Foundation of China(31300555),Special Foundation of Anti-aging Chinese Herbal Research Institution of Anhui Province(2013KSLZX02) and Natural Science Foundation of Anhui Province(KJ2013A207 and 2015KJ004).| Aroca R, Vernieri P, Ruiz-Lozano JM, 2008. Mycorrhizal and non-mycorrhizal Lactuca saliva plants exhibit contrasting res-ponses to exogenous ABA during drought stress and recovery. Journal of Experimental Botany, 59:2029-2041. DOI:10.1093/jxb/ern057. |
| Bates LS, Waldeen RP, Teare ID, 1973. Rapid determination of free proline for water-stress studies. Plant and Soil, 39:205-207. |
| Boogar AR, Salehi H, Jowkar A, 2014. Exogenous nitric oxide alleviates oxidative damage in turfgrasses under drought stress. South African Journal of Botany, 92:78-82. |
| Chen ZL, Li XM, Zhang LH, 2014. Effect of salicylic acid pre-treatment on drought stress responses of Zoysiagrass (Zoysia japonica). Russian Journal of Plant Physiology, 61:619-625. |
| Dhindsa RS, Plumb-Dhindsa P, Thorpe TA, 1981. Leaf senescence:correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32:93-101. |
| Fariduddin Q, Hayat S, Ahmad A, 2003. Salicylic acid influences net photosynthetic rate, carboxylation efficiency nitrate reduc-tase activity, and seed yield in Brassica juncea. Photosynthetica, 41:281-284. |
| Hashempour A, Ghasemnezhad M, Ghazvini RF, et al., 2014. The physiological and biochemical responses to freezing stress of olive plants treated with salicylic acid. Russian Journal of Plant Physiology, 61:443-450. |
| He QQ, Zhao SY, Ma QF, et al., 2014. Endogenous salicylic acid levels and signaling positively regulate arabidopsis response to polyethylene glycol-simulated drought stress. Journal of Plant Growth Regulation, 3:871-880. |
| Heath RL, Packer L, 1968. Photoperoxidation in isolated chlorop-lasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125:189-198. |
| Idrees M, Naeem M, Khan MN, et al., 2012. Alleviation of salt stress in lemongrass by salicylic acid. Protoplasma, 249:709-720. DOI:10.1007/s00709-011-0314-1. |
| Jarvis CE, DuVal A, Crane PR, 2014. Gardenia jasminoides:a traditional Chinese dye plant becomes a garden ornamental in Europe. Curtis's Botanical Magazine, 31:80-89. |
| Kadioglu A, Saruhan N, Sağlam A, et al., 2011. Exogenous salicylic acid alleviates effects of long term drought. Plant Growth Regulation, 64:27-37. DOI:10.1007/s10725-010-9532-3. |
| Knudson LL, Tibbitts TW, Edwards GE, 1977. Measurement of ozone injury by determination of leaf chlorophyll concentra-tion. Plant Physiology, 60:606-608. |
| Li F, Qin XY, Xie YH, et al., 2013. Physiological mechanisms for plant distribution pattern:responses to flooding and drought in three wetland plants from Dongting Lake, China. Limnology, 14:71-76. |
| Liu HY, Wang XD, Wang DH, et al., 2011. Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Industrial Crops and Products, 33:84-88. |
| Lykas C, Petsani D, Kittas C, et al., 2006. Effect of a red to far red light filtering plastic film on growth of gardenia (Gardenia jasminoides). Acta Horticulturae, 711:399-404. |
| Mahmood M, Bidabadi SS, Ghobadi C, et al., 2012. Effect of methyl jasmonate treatments on alleviation of polyethylene glycol-mediated water stress in banana (Musa acuminata cv. 'Berangan', AAA) shoot tip cultures. Plant Growth Regulation, 68:161-169. |
| Michel BE, Kaufmann MR, 1973. The osmotic potential of polye-thylene glycol 6000. Plant Physiology, 51:914-916. |
| Patade VY, Bhargava S, Suprasanna P, 2009. Halopriming imparts tolerance to salt and PEG induced drought stress in sugar-cane. Agriculture, Ecosystems and Environment, 134:24-28. |
| Remy M, Martin E, Hans-Joachim W, 2014. Interactive effects of free-air CO2 enrichment and drought stress on maize growth. European Journal of Agronomy, 52:11-21. |
| Sarunyaporn M, Sittiruk R, Kanyaratt S, 2014. Physiological and comparative proteomic analyses of Thai jasmine rice and two check cultivars in response to drought stress. Journal of Plant Interactions, 9:43-55. |
| Shi GR, Xia SL, Ye J, et al., 2015. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environmental and Experimental Botany, 111:127-134. |
| Singh PK, Gautam S, 2013. Role of salicylic acid on physiological and biochemical mechanism of salinity stress tolerance in plants. Acta Physiologiae Plantarum, 35:2345-2353. |
| Sinhababu A, Kar RK, 2003. Comparative responses of three fuel wood yielding plants to PEG-induced water stress at seedling stage. Acta Physiologiae Plantarum, 25:403-409. |
| Song JL, Wang R, Shi YP, et al., 2014. Iridoids from the flowers of Gardenia jasminoides Ellis and their chemotaxonomic signi-ficance. Biochemical Systematics and Ecology, 56:267-270. |
| Sucre B, 2011. Effect of salinity and PEG-induced water stress on water status, gas exchange, solute accumulation, and leaf growth in Ipomoea pes-caprae. Environmental and Experi-mental Botany, 70:192-203. |
| Velikova V, Yordanov I, Edreva A, 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants:protective role of exogenous polyamines. Plant Science, 151:59-66. |
| Yilmaz DD, Parlak KU, 2011. Nickel-induced changes in lipid peroxidation, antioxidative enzymes, and metal accumulation in Lemna gibba. International Journal of Phytoremediation, 13:805-817. DOI:10.1080/15226514.2010.525563. |
| Zhang HH, Jin WW, Mao WJ, et al., 2012. Effects of exogenous salicylic acid on cell Membrane and chlorophyll fluorescence characteristics in leaves of seedlings under drought stress. Journal of Desert Research, 32:117-121. |
 2016, 8
2016, 8


