Article Information
- YongZhong Su, TingNa Liu, XueFen Wang, Rong Yang. 2016.
- Salinity effects on soil organic carbon and its labile fractions, and nematode communities in irrigated farmlands in an arid region, northwestern China
- Sciences in Cold and Arid Regions, 8(1): 46-53
- http://dx.doi.org/10.3724/SP.J.1226.2016.00046
Article History
- Received: July 21, 2015
- Accepted: September 16, 2015
2. Jinlin Agricultural University, Changchun, Jilin 130118, China
1 Introduction
Salinization is one of the major forms of l and degradation,which threatens sustainable crop production and ecosystem sustainability in many arid and semi-arid regions of the world(Bossio et al., 2007; Chowdhury et al., 2011). Excessive soil salt accumulation affects soil chemical and biological processes,reduces water and nutrient uptake, and limits crop growth, and has been intensively studied(Kenren,2000; Rengasamy,2006; Yang,2008). Over recent decades,it is of particular interest to study the impacts of salinity on soil microbial biomass and activity,carbon and nitrogen mineralization, and biochemical processes essential for maintenance of soil organic matter(Rietz and Haynes, 2003; Yuan et al., 2007; Muhammad et al., 2008; Dendooven et al., 2010; Egamberdieva et al., 2010; Mayi et al., 2012). Some studies indicated that salinity reduces soil microbial biomass and activity,inhibits carbon and nitrogen mineralization and enzyme activities(Beltrán-Hernádez et al., 2007; Tripathi et al., 2007; Yuan et al., 2007; Egamberdieva et al., 2010; Setia et al., 2011). However,contrary results have been reported on the effects of salinity on soil respiration and microbial biomass(Muhammad et al., 2008; Wong et al., 2008,2009). These contradictory observations may be due to differences in soil properties,especially the levels of salinity,soil pH and salt iron composition(Muhammad et al., 2008). Therefore,research about salinity effects on soil biological properties in various soil conditions are still necessary.
Microbial community composition is affected by salinity(Pankhurst et al., 2001; Gennari et al., 2007; Llamas et al., 2008)because microbial genotypes differ in their tolerance to low osmotic potential(Mandeel, 2006; Llamas et al., 2008). Fungi have been reported to be more sensitive to salinity than bacteria(Pankhurst et al., 2001; Wichern et al., 2006) and high salinity in soils may create an unsuitable substrate for the development of fungi in the microbial biomass(Yuan et al., 2007). Change in microbial community composition induced by salinity may affect their predators,such as free-living nematode communities,leading to a change in the prey-predator balance and soil food web structure. Soil nematodes are one of the most abundant groups in soil inhabitants. As the predators of soil microorganisms,soil nematodes occupy a central position in the soil food web(Liang et al., 2005) and regulate the rate of soil organic matter decomposition,nutrient mineralization and cycling(Pen-Mouratov et al., 2003). An underst and ing of soil nematodecommunity response to soil saline change is of importance for interpreting change in the soil food web structure under salt stress and for indicating indirectly the microbial community dynamics as affected by soil salinity. However,little information is available on the effects of salinity on the soil nematode communities(Pen-Mouratov et al., 2011).
The objective of this study was to assess the effect of salinity on SOC and its labile fractions including EOC and MBC, and basal soil respiration(as a measurement of microbial activity); and to determine the characteristics of soil nematode communities along an arid agroecosystem salinity gradient.
2 Materials and methods 2.1 Study areaThis study was conducted in the central section of Linze County,Gansu Province,China. The region,located between 39°10′N-39°25′N and 100°06′E-100°28′E,with an altitude ranging from 1,420 to 1,450 m,is an alluvial plain in the middle reaches of the Heihe River Basin,one of the three largest rivers in the Hexi Corridor. The region has a typical temperate desert climate. Mean annual precipitation within the region encompassing the study site is 117 mm. Mean annual air temperature is 7.6 ℃,with an absolute maximum of 39.1 ℃ and an absolute minimum of −27 ℃. Mean annual pan-evaporation is around 2,390 mm(Su et al., 2007). Saline soils cover an area of about 1.39 million ha in the middle reaches of the Heihe River Basin,accounting to for 3.41% of the total area(Soil Investigation Office in Zhangye Prefecture,Gansu Province,1986) and mostly distributed in the depression,low fluvial terrace in the floodplain and along the border of the alluvial fan. The groundwater table ranged from 1.0 m to 4.0 m. The primary l and scapes of these saline soils were wet grassl and s or saline meadow. In recent three decades,due to population increase,natural saline soils were gradually cultivated. Before cultivation,engineering drainage measures were implemented to reduce groundwater table,i.e.,a drainage channel was excavated about 100 m distance. In cultivation processes,the main ameliorative approach to restrain salt rise was to blend a certain amount of s and s in the surface soils,which can also improve soil structure and tilth. The main crops are maize(Zea mays) and spring wheat(Triticum aestivum Linn.). In the strongly saline fields,salt tolerant crops and forages,sugar beet(Beta vulgaris) and alfalfa(Medicago sativa)were planted.
2.2 Soil samplingIn April 2011,five sites were chosen based on surface salt accumulation status and in situ measurement of electrical conductivity(EC)using a portable electrical conductivity meter. The farml and s of the selected sites are irrigated cropl and s. These cropl and s were affected by various degrees of salinity with different groundwater levels ranging from 1 m to 2 m. In each site,five relatively uniform fields were selected as replicates and ,thus,25 fields were selected. In each field(0.5-1 ha),soil samples were taken from five sub-plots using a soil corer(4 cm diameter×20 cm) and mixed into a pooled sample.
All fresh samples were gently sieved through a 2-mm mesh(visible pieces of crop residues and roots were removed) and a portion of samples was used to analyze microbial biomass C and basal soil respiration incubation and to extract soil nematodes. Another portion of the soil was air-dried for soil particle distribution,pH, and electrical conductivity(EC)analyses, and ground pass a <0.1 mm sieve for SOC,total N, and soil salt analyses.
2.3 Soil analysisSoil particle size distribution was determined by the pipette method in a sedimentation cylinder using sodium hexametaphosphate as the dispersal agent(Gee and Bauder, 1986). Soil organic carbon(SOC)was determined by the Walkley-Black dichromate oxidation method, and total N was measured using the micro-Kjeldahl procedure. Cation exchange capacity(CEC)was determined by flame spectrophotometry after leaching of less than 2-mm air-dried soil with 1 M CH3COONH4 at pH 7.0(ISSCAS,1978).
The ECe(electrical conductivity of the saturated paste extraction)was measured using an electrical conductivity meter(Rhoades,1996). Soil pH was measured using a pH meter at a soil/water ratio of 1:5. CO32− and HCO3− concentrations were measured using the double indicator neutralization method; Cl− concentration was measured using AgNO3 titration and SO42− by EDTA indirect titration. Ca2+ and Mg2+ were measured using EDTA titration and Na+ and K+ were measured using a flame photometer(ISSCAS,1978). The Na+ absorption ratio(SAR)was calculated as: SAR=[Na+]/(0.5[Ca2++Mg2+]1/2).
Easily oxidation organic carbon(EOC)was determined by oxidation with 333 mmol/L KMnO4 according to the method of Blair et al.(1995). Microbial biomass C was estimated by the chloroform fumigation extraction method(Vance et al., 1987). Six portions equivalent to 25 g oven dry soil were taken from each soil sample. Three portions were fumigated for 24 hours at 25 ℃ with ethanol free CHCl3. Following fumigant removal,the soil was extracted with 100 mL 0.5 M K2SO4 by horizontal shaking for 1 hour at 200 rpm and then filtered. The other three non-fumigated portions were extracted simultaneously at the time fumigation commenced. Organic C in the extracts was measured using the dichromate oxidation method(Vance et al., 1987; Yuan et al., 2007). Microbial biomass C was calculated as follows: microbial biomass C = EC/kEC,where EC =(organic C extracted from fumigated soils)−(organic C extracted from non-fumigated soils) and kEC = 0.38.
Basal soil respiration was determined by placing 30 g of field moist soil in a 50 mL beaker and incubating the sample for 7 days in the dark at 25 ℃ in an air tight sealed jar along with 10 mL of 1 M NaOH. The CO2-C evolved was determined after 1,3 and 7 days by titration(Anderson,1982). Basal respiration rate was calculated based on cumulative CO2 -C evolution over the 7 days period.
Soil nematodes were extracted from 100 g soil samples(fresh weight)using a modified cotton-wool filter method(Townshend,1963). After extraction,the nematodes were preserved in a 4% formaldehyde solution for further analysis. The total number of nematodes was recorded, and all the nematodes in each sample were identified to genus level using compound microscopy. The nematode populations were expressed as number of nematodes per 100 g dry soil. Feeding types were classified as bacterivores(Ba),fungivores(Fu),omnivores(Om), and plant-parasitic nematodes(PP)according to Yeates et al.(1993). Characteristics of the nematode communities were described by the following indices: N,total nematode individuals per 100 g dry soil; feeding type composition,recorded as the percentage of bacterivores out of the total number of individuals(Ba%),percentage of fungivores out of the total number of individuals(Fu%),percentage of omnivores out of the total number of individuals(OP%), and percentage of plant-parasitic nematodes out of the total number of individuals(PP%)(Wu et al., 2005); T,trophic diversity,where T=1/∑Pi2 and Pi was the proportion of the i-th trophic group in the nematode community(Herrera,1976).
2.4 Statistical analysisStatistical analyses were performed by SPSS Statistical Software 17.0. One way analysis of variance(ANOVA) and the least significance difference(LSD)test was applied to determine the significant differences in soil parameters between the different salinity levels. The regression analysis was used to evaluate the relationships between soil salinity and other soil properties. Differences obtained at levels of P <0.05 were considered significant.
3 Results 3.1 Soil salt compositionThe soils from the five sites formed a salinity gradient and the total salt content ranged between 1.0±0.1 g/kg and 19.6±3.4 g/kg, and ECe ranged between 1.0±0.3 dS/m and 26.5±5.1 dS/m(Table 1). According to the classification of the Soil Survey Division Staff(1993),the soils from the five sites were categorized into five salinity levels:(1)non-saline(ECe <2),(2)very slightly saline(2≤ ECe <4),(3)slightly saline(4≤ ECe <8),(4)moderately saline(8≤ ECe <16), and (5)strongly saline(ECe ≥16). All soils were non sodic with low SAR values ranging from 0.14 to 1.61. Ca2+ and Mg2+ were dominant in cation components and the proportion of Ca2++Mg2+ as a percentage of cation sum decreased from 95% in the non-saline soils to 84% in the strongly saline soils. SO42− was dominant in anion components and the proportion of Ca2++Mg2+ as anion sum ranged from 32% in non-saline soils to 91% in the strongly saline soils(Table 1).
| Parameter | Non-saline | Very slightly saline | Slightly saline | Moderately saline | Strongly saline |
| ECe (dS/m) | 1.10±0.30 | 3.30±0.60 | 5.80±1.10 | 12.20±2.60 | 26.50±5.10 |
| Total salt (g/kg) | 1.00±0.10 | 6.20±5.80 | 12.50±3.30 | 11.60±2.60 | 19.60±3.40 |
| Na+ (cmol/g) | 0.15±0.06 | 0.85±0.92 | 0.40±0.40 | 3.93±0.39 | 7.56±1.85 |
| K+ (cmol/g) | 0.06±0.01 | 0.22±0.08 | 0.16±0.08 | 0.77±0.63 | 0.82±0.30 |
| Ca2+ (cmol/g) | 0.88±0.15 | 6.57±10.84 | 26.16±0.81 | 13.62±2.79 | 20.30±1.91 |
| Mg2+ (cmol/g) | 1.22±0.21 | 2.84±1.39 | 6.09±2.19 | 12.60±3.66 | 23.82±9.28 |
| HCO3− (cmol/g) | 0.94±0.06 | 0.57±0.06 | 0.49±0.03 | 0.63±0.40 | 0.47±1.23 |
| Cl− (cmol/g) | 0.09±0.02 | 0.27±0.24 | 0.14±0.11 | 1.62±0.36 | 4.55±0.62 |
| SO42− (cmol/g ) | 0.49±0.34 | 9.86±11.61 | 32.73±2.76 | 30.61±8.42 | 50.70±9.97 |
| SAR | 0.14±0.05 | 0.59±0.65 | 0.10±0.10 | 1.23±0.70 | 1.61±0.37 |
Soil particle size distribution indicated that silt was the dominant fraction in all soils. No significant differences in the content of s and ,silt and clay were observed along the salinity gradient. Soil pH values ranged from 8.31 to 8.83, and there were no significant differences among the soils. SOC and total N concentrations were significantly higher in the non-saline soils than in the salt-affected soils and indicated a clear decreasing trend with increasing salinity. There were no significant differences in SOC and total N between the saline soils, and no significant difference in C/N ratio was found. The CEC decreased with increasing salinity(Table 2).
| Parameter | Non-saline | Very slightly saline | Slightly saline | Moderately saline | Strongly saline | ANOVA Significance |
| Sand (%) | 24.80±3.00* | 25.30±3.90 | 26.50±1.70 | 28.90±3.30 | 30.70±5.30 | ns |
| Silt (%) | 49.60±5.20 | 46.50±3.20 | 46.70±2.70 | 43.10±3.70 | 45.00±3.10 | ns |
| Clay (%) | 25.60±3.30 | 31.20±5.70 | 28.80±5.10 | 28.00±3.60 | 24.30±3.10 | ns |
| pH | 8.83±0.74 | 8.31±0.42 | 8.42±0.45 | 8.60±0.19 | 8.71±0.12 | ns |
| SOC (g/kg) | 12.78±1.48 a | 8.27±0.96 b | 8.68±1.60 b | 7.61±1.52 b | 7.03±0.80 b | *** |
| Total N (g/kg) | 1.33±0.18 a | 1.00±0.12 b | 1.09±0.26 b | 0.93±0.18 b | 0.89±0.12 b | *** |
| C/N | 8.90±0.30 | 8.30±0.40 | 8.40±0.50 | 8.20±0.40 | 7.90±0.20 | ns |
| CEC (cmol/g) | 16.27±0.72 a | 13.11±0.97 b | 12.58±1.55 b | 10.79±2.16 c | 9.67±0.78 c | *** |
| * mean±SD followed by different letters show significant differences with different salinity levels using LSD test (P <0.05), *** indicate P <0.001. | ||||||
The microbial biomass C in soil ranged between 70.7 and 164.4 mg/kg,with the greatest value on average occurring in non-saline soils. The percentage of microbial biomass C as organic C ranged from 0.97% to 1.66%. Easily oxidation organic carbon(EOC)ranged between 1.64 g/kg and 3.13 g/kg, and the basal soil respiration varied from 8.6 to 14.1 μg(CO2-C)/(g·d). The microbial biomass C,EOC and basal soil respiration were significantly higher in the non-saline soils than that in the saline soils and showed clear decreasing tread with increasing salinity. Regression analysis indicated that the relationships between ECe and MBC,the percentage of MBC to SOC,EOC and basal soil respiration were best described by power functions(Figure 1).
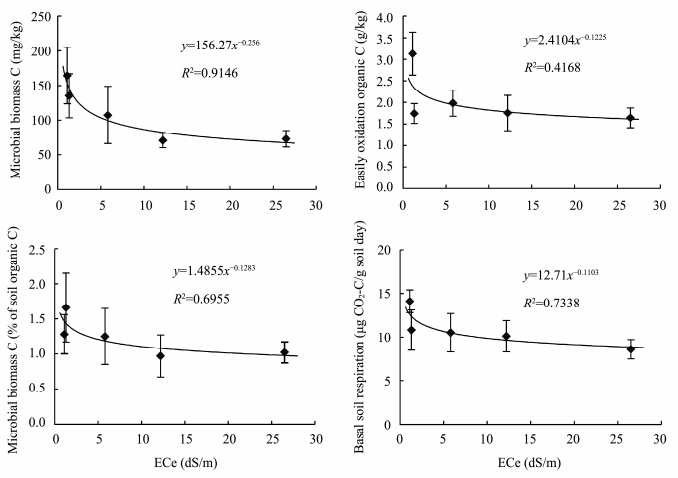 |
| Figure 1 Relationships of ECe with microbial biomass C, the percentage of microbial biomass C as SOC, easily oxidation organic C, and basal soil respiration |
In total,23 nematode genera were identified in the studied soils,including nine plant parasites,fourfungivores,eight bacteriovores and two omnivores-predators. In the non-saline soils,21 nematode genera were recorded,but only 15 nematode genera were found in the strongly saline soils(Table 3).
| Nematode | Non-saline | Very slightly saline | Slightly saline | Moderately saline | Strongly saline | F | P |
| PP | |||||||
| Coslenchus | 29.1±4.6 a | 25.7±6.4 b | 5.2±4.0 c | 13.5±8.4 c | 8.3±3.7 c | 13.860 | <0.0001 |
| Neopsilenchus | 1.2±2.4 | 8.9±6.0 | 10.8±4.4 | 19.1±15.6 | 5.8±4.3 | 2.728 | 0.069 |
| Filenchus | 14.5±9.1 | 23.4±13.2 | 17.0±8.2 | 17.4±4.4 | 3.3±4.1 | 2.760 | 0.067 |
| Psilenchus | 5.5±7.5 | 0.9±1.7 | 2.4±4.9 | 0.0 | 0.0 | 1.308 | 0.312 |
| Tylenchorhynchus | 0.0 b | 9.9±5.2 b | 11.5±6.9 b | 44.0±32.4 a | 0.0 b | 5.841 | 0.005 |
| Helicotylenchus | 12.9±8.8 b | 10.3±12.1 b | 13.7±3.3 b | 38.0±19.8 a | 3.9±0.7 b | 6.813 | 0.002 |
| Pratylenchus | 17.3±10.0 b | 113.2±73.1 a | 48.8±36.1 b | 2.7±5.5 b | 0.0 b | 6.600 | 0.003 |
| Macroposthonia | 0.8±1.6 b | 0.0 b | 14.8±9.7 a | 0.0 b | 0.0 b | 9.000 | 0.001 |
| Paratylenchus | 54.1±35.2 a | 25.1±12.9 b | 8.7±5.2 b | 3.8±2.8 b | 5.5±4.5 b | 6.100 | 0.003 |
| Fu | |||||||
| Tylencholaimus | 0.0 b | 0.0 b | 6.9±13.8 b | 49.0±46.3 a | 32.7±16.5 ab | 3.700 | 0.026 |
| Diphtherophora | 26.2±14.0 ab | 40.6±12.6 a | 16.2±9.5 bc | 11.2±9.0 bc | 4.6±4.9 cd | 7.200 | 0.012 |
| Paraphelenchus | 48.2±17.8 a | 16.7±5.5 b | 40.1±23.6 a | 10.3±5.8 b | 11.8±4.6 b | 6.400 | 0.003 |
| Aphelenchoides | 55.4±26.2 a | 55.8±37.2 a | 49.9±26.8 a | 10.5±6.9 b | 12.2±7.2 b | 3.800 | 0.026 |
| Ba | |||||||
| Mesorhabditis | 17.1±8.7 ab | 22.3±8.5 a | 8.6±4.2 b | 0.0 c | 0.0 c | 12.150 | <0.0001 |
| Ablechroiulus | 19.4±8.9 a | 0.0 b | 0.0 b | 0.0 b | 0.0 b | 18.867 | <0.0001 |
| Eucephalobus | 18.1±11.2 b | 7.4±5.8 b | 135.4±39.5 a | 95.2±43.1 a | 24.1±14.5 b | 16.700 | <0.0001 |
| Acrobeles | 11.0±7.3 b | 5.9±6.9 b | 150.3±53.9 a | 15.7±6.8 b | 4.2±3.6 b | 26.126 | <0.0001 |
| Acrobeloides | 33.2±16.2 a | 10.5±12.2 b | 8.4±9.9 b | 6.8±13.6 b | 2.6±3.1 b | 4.112 | 0.019 |
| Eumonhystera | 9.5±4.8 a | 7.1±6.7 a | 1.4±2.8 b | 0.0 b | 0.0 b | 5.183 | 0.008 |
| Plectus | 68.3±38.8 | 78.0±62.1 | 121.2±62.1 | 106.7±96.4 | 60.6±15.0 | 0.740 | 0.579 |
| Prismatolaimus | 16.9±13.0 | 3.4±5.7 | 4.4±6.5 | 0.3±0.6 | 1.9±1.4 | 3.566 | 0.031 |
| Om | |||||||
| Epidorylaimus | 13.7±14.3 | 0.0 | 0.0 | 4.4±5.4 | 9.1±3.3 | 2.855 | 0.061 |
| Aporcelaimium | 4.6±5.3 | 0.0 | 16.7±19.8 | 0.0 | 0.0 | 2.482 | 0.088 |
| Ecological indices | |||||||
| Total number | 477.1±99.7 a | 465.0±217.9 a | 692.4±247.8 a | 448.7±186.6 a | 190.5±49.0 b | 4.069 | 0.020 |
| Trophic diversity (T) | 3.1±0.3 a | 2.7±0.2 ab | 2.2±0.2 c | 2.5±0.3 bc | 2.6±0.3 bc | 5.406 | 0.007 |
| Values are presented as mean±SD (n=5). Ba, Fu, Om and PP represent bacterivores, fungivores, omnivores and plant-parasitic nematodes, respectively. Values followed by the different letter within rows are significantly different at P <0.05. | |||||||
Nematode abundance per 100 g dry soil showed considerable variation in soils from different salinity levels,ranging from 692.4±247.8 in the slightly saline soil to 190.3±49.0 in the strongly saline soil(Table 3). The nematode abundance was significantly lower in the strongly saline soil than in the other soils,but no significant differences in total nematode abundances were observed among the non-saline,slight and moderate soils. There were significant differences for the abundance of most nematode genera between the soils in different salinity levels,but clear change trends along salinity gradient were not found(Table 3).
The percentage of trophic groups are presented in Figure 2. Bacterivores were the most abundant trophic group in the soils except for the very slightly saline soil,with the highest percentage of 62% of trophic groups. Also,bacterivores occupied about 50% of trophic groupsin the moderately and strongly saline soils. The omnivores-predators were the least abundant trophic group and occupied 0.0%-4.8% of trophic groupsin the soils.The trophic diversity(T)ranged from 2.20 to 3.07,with the greatest and lowest values in the non saline soil and slightly saline soil,respectively(Table 3).
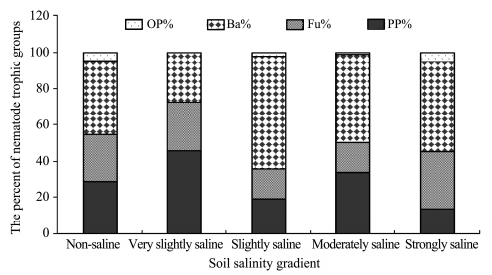 |
| Figure 2 Soil nematode composition of trophic groups in soils of salinity gradient. Ba, Fu, PP and OP refer to bacteriovores, fungivores, plant parasites and omnivores-predators respectively |
In this study,the sampling fields were converted from natural salinization wet grassl and s to cropl and s. In the long-term agricultural use,salt content at the tillage layer gradually decreased through effective measures including channel drainage,manure application,blending s and s into surface layer, and planting salt-tolerant crops such as sugar beet(Liu et al., 2010). However,due to strong evaporation in this arid region and frequent rise and decline in groundwater level under different seasons,salt accumulation at the top soil is still distinct. The salinity of soils is characterized by distinct small scale variability and strong fluctuations throughout the year. In this study,the samples were taken during salt accumulation in the topsoil period in the spring so that the electrical conductivity and soluble content presented here reflected the site-specific maximum salt concentration.
The results show that SOC,total N and CEC decreased with increasing salinity and the average SOC concentrations were below 10 g/kg in salt-affected soils,which was in agreement with the result from Yuan et al.(2007)who suggested that salinity-induced degradation in arid soils is characterized by low SOC concentrations. The increased salinity also led to a decline in labile organic C fractions including microbial biomass C and easily oxidation organic C,suggesting a negative effect of increasing salinity on the soil microbial community(Rietz and Haynes, 2003). The decreased labile organic C fractions also suggested that the decomposition of organic matter and release of nutrients required to sustain soil productivity were inhibited by high salinity(Ghollarata and Raiesi, 2007; Tripathi et al., 2007) and the availability of soil organic matter to soil microorganisms was reduced(Muhammad et al., 2008). Since soil basal respiration represents the living component of microbial biomass C(Sparling,1992),it showed a similar trend along salinity gradient to microbial biomass C. In the present study,the microbial biomass C to SOC ratios ranged from 0.97% to 1.66% and was far below the values suggested by Anderson and Domsch(1989) and Sparling(1992): about 4% of soil organic C belongs to the microbial biomass. Similar results have been reported by Yuan et al.(2007)in arid regions. The relatively low microbial biomass C,CO2-C emission rate and the microbial biomass C to SOC ratios in saline soils in the present study indicated a low substrate utilization efficiency caused by high salinity(Yuan et al., 2007).
Soil nematode have been used as an indicator of soil conditions with an underlying assumption that larger,more diverse community composition and abundance reflects more healthy soils(Yeates,2003). Pen-Mouratov et al.(2011)found that the free-living nematode community exhibited a negative correlation with electrical conductivity. In the present study,although the nematode communities and abundance show high variability in different soils,there was a significantly negative relationship between ECe and the total nematode abundance(Figure 3),indicating that soil salinity was one of the important factors to affect nematode community composition and abundance. The total nematode abundance was significantly lower in the strongly saline soils than in the other soils,suggesting that excessive amounts of salts inhibited the existence of some nematode species. No significant difference in the total nematode abundance was observed among non-saline,slightly saline and moderately saline soils. This indicated that slight and moderate salinity did not exert significant stress to the nematode communities. Nematodes are now accepted as playing critical roles in controlling the turnover of the soil microbial biomass and thus in the availability of plant nutrients(Yeates,2003). The low soil microbial biomass C and basal respiration in the strongly saline soils may be associated with the reduced nematode abundance.
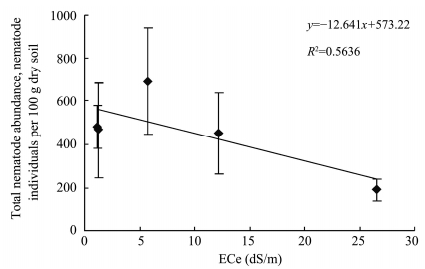 |
| Figure 3 Relationship of ECe with total nematode abundance |
Nematode population dynamics provides an insight into the availability of food resources and the communities upon which they feed(Yeates and Williams, 2001). In the present study,changes in nematode trophic groups should closely link with microbial community dynamics as affected by soil salinity. Bacterial communities have been shown to be dominate in salt-affected soils(Kazunori and Oba, 1994; Pankhurst et al., 2001). The present study shows that bacterivores were the largest trophic group in the slightly,moderately and strongly saline soils,which indicated that the principal food resource for nematodes was bacteria, and the energy pathway was mainly dominated by bacteria in salt-affected soils. Soil salinity resulted in a decrease of the trophic diversity(T)of nematode communities and the lowest trophic diversity in the slightly saline soils indicated a sensitivity of nematode trophic groups to soil conditions at this salinity stage.
5 ConclusionsThis study demonstrated that soil salinity has negative impact on SOC,labile organic C fractions including easily oxidation organic C and microbial biomass C, and CO2-C emission. Excessive salt accumulation led to a decline in soil nematode community diversity and abundance, and change in nematode trophic group. Some effective measures such as application of manure and planting salt-tolerant forage such as alfalfa in agricultural management should be taken into account to increase SOC level and restrain soil salinity. Further study is needed to determine the response and acclimation of soil nematodes to saline stress and to identify the interactions between soil microbial communities,soil nematode community and diversity, and soil salinity from the relationship of prey-predator.
Acknowledgments: This research was supported by the National Natural Science Foundation of China(91425302,41401337).| Anderson JPE, 1982. Soil Respiration. In:Page AL (ed.). Methods of Soil Analysis, Part 2. Chemical and Microbiological Prop-erties. Soil Science Society of America, Madison, Wisconsin, pp. 837-871. |
| Anderson TH, Domsch KH, 1989. Ratios of microbial biomass to total organic carbon in arable soils. Soil Biology & Biochemistry, 21:471-479. |
| Beltrán-Hernádez RI, Luna-Guido ML, Dendooven L, 2007. Emission of carbon dioxide and dynamics of inorganic N in a gradient of alkaline soils of the former lake Texcoco. Applied Soil Ecology, 35:390-403. |
| Blair GJ, Lefroy RDB, Lisle L, 1995. Soil carbon fractions, based on their degree of oxidation and the development of a carbon management index for agricultural systems. Australian Journal of Soil Research, 46:1459-1466. |
| Bossio D, Critchley W, Geheb K, et al., 2007. Conserving soil-protecting water. In:Comprehensive Assessment of Water Management in Agriculture:Water for Food, Water for Life. Stylus Publishing, LLC, Sterling, VA, pp. 551-584. |
| Chowdhury N, Marschner P, Burns RG, 2011. Soil microbial activity and community composition:impact of changes in matric and osmotic potential. Soil Biology and Biochemistry, 43:1229-1236. |
| Dendooven L, Alcántara-Hernández RJ, Valenzuela-Encinas C, et al., 2010. Dynamics of carbon and nitrogen in an extreme alkaline saline soil:A review. Soil Biology & Biochemistry, 42:865-877. |
| Egamberdieva D, Renella G, Wirth S, et al., 2010. Secondary salinity effects on soil microbial biomass. Biology and Fertility of Soils, 46:445-449. |
| Gee WG, Bauder JW, 1986. Particle-size analysis. In:Klute A (ed.). Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. American Society of Agronomy, Soil Science Society of America, Madison, WI, USA, pp. 383-412. |
| Gennari M, Abbate C, La Porta V, et al., 2007. Microbial response to Na2SO4 additions in a volcanic soil. Arid Land Research and Management, 21:211-227. |
| Ghollarata M, Raiesi F, 2007. The adverse effects of soil salinization on the growth of Trifolium alexandrinum L. and associated microbial and biochemical properties in a soil from Iran. Soil Biology & Biochemistry, 39:1699-1702. |
| Herrera CM, 1976. A trophic diversity index for presence-absense food data. Oecologia, 25:187-191. |
| Institute of Soil Sciences, Chinese Academy of Sciences (ISSCAS), 1978. Physical and Chemical Analysis Methods of Soils. Shanghai, China:Shanghai Science Technology Press. |
| Kazunori S, Oba Y, 1994. Effect of fungal to bacterial biomass ratio on the relationship between CO2 evolution and total soil microbial biomass. Biology and Fertility of Soils, 17:39-44. |
| Kenren R, 2000. Salinity. In:Sumner ME (ed.). Handbook of Soil Science. Boca Raton:CRC Press, pp. 3-25. |
| Liang WJ, Li Q, Jiang Y, et al., 2005. Nematode faunal analysis in an aquic brown soil fertilized with slow-release urea, Northeast China. Applied Soil Ecology, 29:285-292. |
| Liu WJ, Su YZ, Yang R, et al., 2010. Land use effects on soil organic carbon, nitrogen and salinity in saline-alkaline wetland. Sciences in Cold and Arid Regions, 2(3):263-270. |
| Llamas DP, Gonzales MD, Gonzales CI, et al., 2008. Effects of water potential on spore germination and viability of Fusarium species. Journal of Industrial Microbiology & Biotechnology, 35:1411-1418. |
| Mandeel QA, 2006. Biodiversity of the genus Fusarium in saline soil habitats. Journal of Basic Microbiology, 46:480-494. |
| Mayi MS, Marschner P, Chittleboroug DJ, et al., 2012. Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biology & Biochemistry, 45:8-13. |
| Muhammad S, Müller T, Joergensen GR, 2008. Relationships between soil biological and other soil properties in saline and alkaline arable soils from the Pakistani Punjab. Journal of Arid Environments, 21:448-457. |
| Pankhurst CE, Yu S, Hawke BG, et al., 2001. Capacity of fatty acid profiles and substrate utilisation patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations win South Australia. Biology and Fertility of Soils, 33:204-217. |
| Pen-Mouratov S, Hu C, Hindin E, et al., 2011. Soil microbial activity and a free-living nematode community in the playa and in the sandy biological crust. Biology and Fertility of Soils, 47:363-375. |
| Pen-Mouratov S, Rakhimbaev M, Steinberger Y, 2003. Seasonal and spatial variation in nematode communities in a Negev desert ecosystem. Journal of Nematology, 35:157-166. |
| Rengasamy P, 2006. World salinization with emphasis on Australia. Journal of Experimental Botany, 57:1017-1023. |
| Rhoades JD, 1996. Salinity:Electrical conductivity and total dis-solved salts. In:Sparks DL, Page AL, Helmke PA, et al. (ed.). Methods of Soil Analysis. Part 3. SSSA Book Series No. 5. Madison, WI:ASA and SSSA, pp. 417-436. |
| Rietz DN, Haynes RJ, 2003. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biology & Biochemistry, 35:845-854. |
| Setia R, Marschner P, Baldock J, et al., 2011. Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biology & Biochemistry, 43:667-674. |
| Soil Investigation Office in Zhangye Prefecture, Gansu Province, 1986. Soils in Zhangye Prefecture, Gansu, pp. 161-171. |
| Soil Survey Division Staff, 1993. Soil Survey Manual. USDA Handbook vol. 18. US Government Printing Office, Washington D.C.. |
| Sparling GP, 1992. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Australian Journal of Soil Research, 30:195-207. |
| Su YZ, Zhao WZ, Su PX, et al., 2007. Ecological effects of desertification control and desertified land reclamation in an oasis-desert ecotone in an arid region:A case study in Hexi Corridor, northwest China. Ecological Engineering, 29:117-124. |
| Townshend JL, 1963. A modification and evaluation of the apparatus for the Oostenbrink direct cotton wool filter extraction method. Nematologica, 9:106-110. |
| Tripathi S, Chakraborty A, Chakrabarti K, et al., 2007. Enzyme activities and microbial biomass in coastal soils of India. Soil Biology & Biochemistry, 39:2840-2848. |
| Vance ED, Brookes PC, Jenkinson DS, 1987. An extraction method for measuring soil microbial biomass C. Soil Biology & Bio-chemistry, 19:703-707. |
| Wichern J, Wichern F, Joergensen RG, 2006. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma, 137:100-108. |
| Wong VNL, Dalal RC, Greene RSB, 2008. Salinity and sodicity effects on respiration and microbial biomass of soil. Biology and Fertility of Soils, 44:943-953. |
| Wong VNL, Dalal RC, Greene RSB, 2009. Carbon dynamics of sodic and saline soils following gypsum and organic material additions:A laboratory incubation. Applied Soil Ecology, 41:29-40. |
| Wu JH, Fu CZ, Lu F, et al., 2005. Changes in free-living nematode community structure in relation to progressive land reclamation at an intertidal marsh. Applied Soil Ecology, 9:47-58. |
| Yang JS, 2008. Development and prospect of the research on salt-affected soils in China. Acta Pedologica Sinica, 45:838-845. |
| Yeates, 2003. Nematodes as soil indicators:functional and biodiversity aspects. Biology and Fertility of Soils, 37:199-210. |
| Yeates GW, Bongers T, De Goede RGM, et al., 1993. Feeding habits in soil nematode families and genera:an outline for soil ecologists. Journal of Nematology, 25:315-331. |
| Yeates GW, Williams PA, 2001. Influence of three invasive weeds pp. 837-871. |
| Anderson TH, Domsch KH, 1989. Ratios of microbial biomass to total organic carbon in arable soils. Soil Biology & Biochemistry, 21:471-479. |
| Beltrán-Hernádez RI, Luna-Guido ML, Dendooven L, 2007. Emission of carbon dioxide and dynamics of inorganic N in a gradient of alkaline soils of the former lake Texcoco. Applied Soil Ecology, 35:390-403. |
| Blair GJ, Lefroy RDB, Lisle L, 1995. Soil carbon fractions, based on their degree of oxidation and the development of a carbon management index for agricultural systems. Australian Journal of Soil Research, 46:1459-1466. |
| Bossio D, Critchley W, Geheb K, et al., 2007. Conserving soil-protecting water. In:Comprehensive Assessment of Water Management in Agriculture:Water for Food, Water for Life. Stylus Publishing, LLC, Sterling, VA, pp. 551-584. |
| Chowdhury N, Marschner P, Burns RG, 2011. Soil microbial activity and community composition:impact of changes in matric and osmotic potential. Soil Biology and Biochemistry, 43:1229-1236. |
| Dendooven L, Alcántara-Hernández RJ, Valenzuela-Encinas C, et al., 2010. Dynamics of carbon and nitrogen in an extreme alkaline saline soil:A review. Soil Biology & Biochemistry, 42:865-877. |
| Egamberdieva D, Renella G, Wirth S, et al., 2010. Secondary salinity effects on soil microbial biomass. Biology and Fertility of Soils, 46:445-449. |
| Gee WG, Bauder JW, 1986. Particle-size analysis. In:Klute A (ed.). Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. American Society of Agronomy, Soil Science Society of America, Madison, WI, USA, pp. 383-412. |
| Gennari M, Abbate C, La Porta V, et al., 2007. Microbial response to Na2SO4 additions in a volcanic soil. Arid Land Research and Management, 21:211-227. |
| Ghollarata M, Raiesi F, 2007. The adverse effects of soil salinization on the growth of Trifolium alexandrinum L. and associated microbial and biochemical properties in a soil from Iran. Soil Biology & Biochemistry, 39:1699-1702. |
| Herrera CM, 1976. A trophic diversity index for presence-absense food data. Oecologia, 25:187-191. |
| Institute of Soil Sciences, Chinese Academy of Sciences (ISSCAS), 1978. Physical and Chemical Analysis Methods of Soils. Shanghai, China:Shanghai Science Technology Press. |
| Kazunori S, Oba Y, 1994. Effect of fungal to bacterial biomass ratio on the relationship between CO2 evolution and total soil microbial biomass. Biology and Fertility of Soils, 17:39-44. |
| Kenren R, 2000. Salinity. In:Sumner ME (ed.). Handbook of Soil Science. Boca Raton:CRC Press, pp. 3-25. |
| Liang WJ, Li Q, Jiang Y, et al., 2005. Nematode faunal analysis in an aquic brown soil fertilized with slow-release urea, Northeast China. Applied Soil Ecology, 29:285-292. |
| Liu WJ, Su YZ, Yang R, et al., 2010. Land use effects on soil organic carbon, nitrogen and salinity in saline-alkaline wetland. Sciences in Cold and Arid Regions, 2(3):263-270. |
| Llamas DP, Gonzales MD, Gonzales CI, et al., 2008. Effects of water potential on spore germination and viability of Fusarium species. Journal of Industrial Microbiology & Biotechnology, 35:1411-1418. |
| Mandeel QA, 2006. Biodiversity of the genus Fusarium in saline soil habitats. Journal of Basic Microbiology, 46:480-494. |
| Mayi MS, Marschner P, Chittleboroug DJ, et al., 2012. Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biology & Biochemistry, 45:8-13. |
| Muhammad S, Müller T, Joergensen GR, 2008. Relationships between soil biological and other soil properties in saline and alkaline arable soils from the Pakistani Punjab. Journal of Arid Environments, 21:448-457. |
| Pankhurst CE, Yu S, Hawke BG, et al., 2001. Capacity of fatty acid profiles and substrate utilisation patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations win South Australia. Biology and Fertility of Soils, 33:204-217. |
| Pen-Mouratov S, Hu C, Hindin E, et al., 2011. Soil microbial activity and a free-living nematode community in the playa and in the sandy biological crust. Biology and Fertility of Soils, 47:363-375. |
| Pen-Mouratov S, Rakhimbaev M, Steinberger Y, 2003. Seasonal and spatial variation in nematode communities in a Negev desert ecosystem. Journal of Nematology, 35:157-166. |
| Rengasamy P, 2006. World salinization with emphasis on Australia. Journal of Experimental Botany, 57:1017-1023. |
| Rhoades JD, 1996. Salinity:Electrical conductivity and total dis-solved salts. In:Sparks DL, Page AL, Helmke PA, et al. (ed.). Methods of Soil Analysis. Part 3. SSSA Book Series No. 5. Madison, WI:ASA and SSSA, pp. 417-436. |
| Rietz DN, Haynes RJ, 2003. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biology & Biochemistry, 35:845-854. |
| Setia R, Marschner P, Baldock J, et al., 2011. Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biology & Biochemistry, 43:667-674. |
| Soil Investigation Office in Zhangye Prefecture, Gansu Province, 1986. Soils in Zhangye Prefecture, Gansu, pp. 161-171. |
| Soil Survey Division Staff, 1993. Soil Survey Manual. USDA Handbook vol. 18. US Government Printing Office, Washington D.C.. |
| Sparling GP, 1992. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Australian Journal of Soil Research, 30:195-207. |
| Su YZ, Zhao WZ, Su PX, et al., 2007. Ecological effects of desertification control and desertified land reclamation in an oasis-desert ecotone in an arid region:A case study in Hexi Corridor, northwest China. Ecological Engineering, 29:117-124. |
| Townshend JL, 1963. A modification and evaluation of the apparatus for the Oostenbrink direct cotton wool filter extraction method. Nematologica, 9:106-110. |
| Tripathi S, Chakraborty A, Chakrabarti K, et al., 2007. Enzyme activities and microbial biomass in coastal soils of India. Soil Biology & Biochemistry, 39:2840-2848. |
| Vance ED, Brookes PC, Jenkinson DS, 1987. An extraction method for measuring soil microbial biomass C. Soil Biology & Bio-chemistry, 19:703-707. |
| Wichern J, Wichern F, Joergensen RG, 2006. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma, 137:100-108. |
| Wong VNL, Dalal RC, Greene RSB, 2008. Salinity and sodicity effects on respiration and microbial biomass of soil. Biology and Fertility of Soils, 44:943-953. |
| Wong VNL, Dalal RC, Greene RSB, 2009. Carbon dynamics of sodic and saline soils following gypsum and organic material additions:A laboratory incubation. Applied Soil Ecology, 41:29-40. |
| Wu JH, Fu CZ, Lu F, et al., 2005. Changes in free-living nematode community structure in relation to progressive land reclamation at an intertidal marsh. Applied Soil Ecology, 9:47-58. |
| Yang JS, 2008. Development and prospect of the research on salt-affected soils in China. Acta Pedologica Sinica, 45:838-845. |
| Yeates, 2003. Nematodes as soil indicators:functional and biodiversity aspects. Biology and Fertility of Soils, 37:199-210. |
| Yeates GW, Bongers T, De Goede RGM, et al., 1993. Feeding habits in soil nematode families and genera:an outline for soil ecologists. Journal of Nematology, 25:315-331. |
| Yeates GW, Williams PA, 2001. Influence of three invasive weeds and site factors on soil microfauna in New Zealand. Pedobiologia, 45:367-383. |
| Yuan BC, Li ZZ, Liu H, et al., 2007. Microbial biomass and activity in salt affected soils under arid conditions. Applied Soil Ecology, 35:319-328. |
 2016, 8
2016, 8


