Article Information
- Akinwumi J. Akinloye, Temitope I. Borokini, Kehinde A. Adeniji, Funmilola M. Akinnubi. 2015.
- Comparative anatomical studies of Artocarpus altilis(Parkinson) Fosberg and Artocarpus communis(J. R. &G. Forster) in Nigeria
- Sciences in Cold and Arid Regions, 7(6): 709-721
- http://dx.doi.org/10.3724/SP.J.1226.2015.00709
Article History
- Received: October 10, 2014
- Accepted: May 15, 2015
2. Plant Genetic Resource Unit, National Centre for Genetic Resources and Biotechnology, Ibadan, Oyo State, Nigeria;
3. Forestry Research Institute of Nigeria, P.M.B. 5054, Jericho, Ibadan, Oyo state, Nigeria
1 Introduction
Breadfruit belongs to the genus Artocarpus(Moraceae), which consists of approximately 60 species native to the Indian subcontinent, Southeast Asia, and Australasia(Jarrett, 1959a, b; Kochummen, 2000). The genus name Artocarpus is derived from the Greek Word 'Artos', bread and 'karpus' which refer to the bread like quality of breadfruit when baked. The tree is found throughout the tropics and cultivated on most Pacific Isl and s. The trees are monoecious, with male and female flowers growing on the same tree, mostly pollinated by wind(Jarrett, 1959a, b; Brantjes, 1981). They grow to heights of 15 to 21 m or more and the trunks may be as large as 2 m in diameter at the base. The trees begin fruit bearing in 3-5 years and are productive for many decades. Single-trunked trees grow with a spreading, evergreen canopy. Leaves are alternate, broadly obovate to broadly ovate, almost entire, with only slight lobing to deeply pinnately lobed, with sinuses up to 2/3 or more of the distance from margin to midrib, with up to six pairs of lobes and a large apical tip. Leaf blade is generally smooth, glossy, dark green with green or yellow-green veins, and few to many white to reddish-white trichomes on the midrib and veins. Leaves on new shoots and root suckers are generally larger and more hirsute than leaves on mature branches. Size is variable depending on the variety, ranging in 15-60 cm long. Fruits are variable in shape, size, and surface texture(Figure 1). They are usually round, oval, or oblong ranging from 9 to 20 cm wide and more than 30 cm long, weighing 0.25-6.00 kg.
 |
| Figure 1 Fruits of A. communis(a) and A. altilis(b) |
Seeded breadfruit(Figure 2a)produces abundant nutritious fruits that are typically consumed as a starchy staple when firm and mature. Seeds are high in protein and low in fat and a good source of vitamins and minerals. All parts are used medicinally in the Pacific and Caribbean, especially the latex, leaf tips, and inner bark. The latex is massaged into the skin to treat broken bones and is b and aged on the spine to relieve sciatica. It is commonly used to treat skin ailments and fungus diseases such as "thrush", which is also treated with crushed leaves. Diluted latex is taken internally to treat diarrhea, stomach aches, and dysentery. The sap from crushed stems and leaves is used to treat ear infections or sore eyes. The root is astringent and used as a purgative; when macerated it is used as a poultice for skin ailments. The bark is also used to treat headaches in several isl and s. In the West Indies, the yellow leaf is brewed into tea and taken to reduce high blood pressure and relieve asthma. The tea is also thought to control diabetes.
 |
| Figure 2 Seeded fruit of A. communis(a) and seedless fruit of A. atilis(b) |
The seeds are edible and are of high nutritional value(Keay et al.,1989). When the seeds are cooked, they are a fair source of thiamine and vitamin C(Amusa et al.,2002). The seeds could be cooked for the main dish, roasted for snacks or even converted to flour, which can be used for snacks or as soup thickener(Anazonwu-Bello, 1986).
In Nigeria, seedless breadfruit(Figure 2b)is regarded as the poor man's substitute for yam(Dioscorea esculenta(Lour.)Burkill and D. cayenensis Lam.)due to the fact that it is used in several traditional food preparations in replacement for yam and also costs <1/3 the price of yams at the market(Mayaki et al.,2003).
Among the Yoruba tribe of Nigeria, it is pounded into a paste, like pounded yam and eaten with various types of soups. Sometimes, it is boiled and eaten like yams. It is prepared into flour and reconstituted with hot water into paste and eaten with soup. The flour is reconstituted with water and fried into puff-puff. When the ripe fruit is used it is highly sugary. The fruit is also peeled and fried into chips.
Breadfruit exhibits great morphological variability, ranging from true seedless varieties to those with several small aborted seeds, or one to a few viable seeds, to varieties with numerous viable seeds.
Two varieties, seeded and seedless fruits, were observed for breadfruit. Since many cultivars of breadfruit are seedless, it has been inferred that fruit development is due to parthenocarpy(Barrau, 1976). Hasan and Razak(1992)showed that the fruits of breadfruit develop normally without pollination. Data available for seeded breadfruit basically indicate diploidy with 2n = 2x = 56; seedless cultivars are commonly triploid with 2n = 3x = 84(Jarrett, 1959b; Barrau, 1976). Ragone(2001)attributed the loss of fertility in sterile diploid and triploid breadfruit to hybridization. Zerega et al.(2005)noted much confusion in the systematics of breadfruit with regards to the binomial, demonstrated by inconsistency regarding the correct name and specific epithet. Furthermore, the species delimitations within the breadfruit is complex that includes up to three species, A. altilis(domesticated breadfruit), A. mariannensis Tre'cul, and A. camanis Lanco, which pose a huge task in plant systematics. Morphological diversity is partitioned differently among these species according to various authors. Jarrett(1959b)published the most recent treatment for the breadfruit complex and took a conservative approach, recognizing one highly variable species, A. communis, which encompasses the diversity represented by both domesticated breadfruit and its closest relatives. However, Jarrett(1959b)acknowledged that the material examined was inadequate and mostly sterile, and suggested that further detailed studies were necessary.
This study was conducted to investigate the anatomical features in the seeded and seedless breadfruit with the view of assessing the species delimitation and providing useful taxonomic information about the taxa.
2 Materials and methods 2.1 Collection and preparation of plant samplesSamples of mature root, wood, leaves and fruits of both A. communis(seeded) and A. altilis(seedless)were collected on the campus of Obafemi Awolowo University, Osun State, Nigeria(7°31'9.86''N; 4°31'34.23"E). The collected samples were preserved in fixative of 50% ethanol.
2.2 SectioningTransverse(TS), tangential longitudinal(TLS) and radial longitudinal sections(RLS)of the wood and root as well as the TS of leaf and petiole of each plant were cut at 10 micron thickness. All the sections were done using a sledge microtome(Reichert, Austria).
2.3 Leaf peelingMature leaves were cut into sizeable portions and kept in concentrated nitric acid until the surfaces becomes swollen under the hot sun. The pieces of leaves with swollen surfaces were transferred into water in a glass petri dish. The swollen surfaces were peeled off using fine tip forceps. The peels were preserved in 50% ethanol.
2.4 Leaf clearingMatured leaves were cut into sizeable portions and boiled in absolute ethanol for forty minutes, rinsed in water twice and soaked in 5% sodium hydroxide for 16 hours. After rinsed in water twice, they were transferred into 5% domestic bleach(JIK). They remained in the bleach until the whole leaves became completely white. They were then rinsed in water thrice and preserved in 50% ethanol.
2.5 Staining of sectionsThe wood, root, leaf and petiole sections were stained for three minutes in Safranin O, rinsed in water twice and counter stained for three minutes in Alcian blue and again rinsed in water twice. The washed and counter stained sections were treated in a series of ethanol solution(50%, 70%, 80%, 90%, and 100%)to remove water molecules(dehydration process) and to remove excess stain(differentiation process). The dehydrated and differentiated sections were transferred into absolute xylene in two series to remove the last trace of water, to clear the sections(making it more transparent) and to remove the last trace of ethanol. Each section was then mounted on a glass slide in DPX(R) mountant.
2.6 Maceration and staining of wood and root maceratesStem and root wood from each species were sliced into small pieces using a pen knife and macerated using Schulze's fluid obtained by mixing equal volume of 10% chromic acid [dissolved 1 g potassium nitrate(KNO3)in 50 mL concentrated nitric acid(HNO3)] and 10% nitric acid. The maceration was carried out in a beaker and kept in the oven at 90 °C for three hours. The macerated wood samples were washed in five changes of water and stained for three minutes in Safranin O then mounted in 25% glycerol on a glass slide and covered with a glass cover slip. The edges of the cover slip were sealed to the slide using nail polish.
2.7 Staining of peels and clear leavesThe epidermal peels and cleared leaves of the Artocarpus species were stained for three minutes in Safranin O. They were rinsed thrice in water and mounted in 25% glycerol on a glass slides and covered with a glass cover slip, whose edges were sealed with nail polish.
2.8 MicroscopyMicroscopic observation of each slide was made and recorded. Photo micrographs of the slides were made using an Accu-scope trinocular microcope(ACCU-scope 3001 LED Trinocular microscope with 3.2 MP CMOS digital camera). Tissues and cells identification and description of wood samples of the Artocarpus species was done according to IAWA hardwood features list, definition and illustration(IAWA, 1989).
Tissues and cell identification and description of leaf and petiole was done according to Esau(1977), Fahn(1977), Cutter(1978), Bilgrami et al.(1983), and Metcalfe and chalk(1989).
3 Results1)There are striking similarities in the macromorphology(Figure 3) and the sections of the root wood, stem bark, root bark, leaf, petiole and leaf macromorphological characters of A. altilis and A. communis. However, in spite of the similarities, several differences were also observed among the two species. The xylem vessels in the stem wood of both species are described as diffuse porous system, with presence of few tyloses; xylem parenchyma is made up of apotracheal parenchyma; rays are homogenous and procumbent and uniseriate ray scanty(Table 1, Figure 4). Furthermore, stem wood fibres were non-septate, non-storeyed and no pitting in both species with large lumen; starch granules were present in the wood and crystal druses were also observed in the parenchyma cells of the pith and phloem(Table 1, Figure 4). However, vessel length differs in both species, being 379±1 µm in A. altilis and 184±1 µm in A. communis(Table 1). In addition, the stem wood fibre maceration show differences in lumen size, wall thickness, fibre diameter and fibre length between samples of the two species(Table 1, Figures 5, 6). Also, root wood of both species show a diffuse porous xylem vessel system, simple pitting on the vessel element, apotracheal type of xylem parenchyma, non-storeyed, homogenous and procumbent rays and non-septate root wood fibre with large lumen(Table 2). Prismatic crystals were observed in the root of A. altilis while crystal druses were seen in A. communis(Table 3, Figures 7, 8).
 |
| Figure 3 Abaxial leaf surfaces of A. altilis(a) and A. communis(d), adaxial leaf surfaces of A. altilis(b) and A. communis(e), and leaf margins of A. altilis(c) and A. communis(f) |
| Characters | A. altilis | A. communis |
| Xylem vessel | Diffuse porous system, solitary vessel predominant, pore multiple 2-15, pore cluster 2-5. Tyloses present in few vessels | Diffuse porous system, solitary vessel predominant, pore multiple 2-20, pore cluster 2-5. Tyloses present in few vessels |
| Vessel element | Simple pitting alternate very small, perforation plate simple and scalariform, some vessels have short tail either at both ends or one end while others have no tail at all. Average vessel length 379±1 µm. Spiral ring present | Simple pitting alternate very small, perforation plate simple and scalariform, some vessels have short tail either at both ends or one end while others have none Average vessel length 184±1 µm. Spiral ring present |
| Xylem parenchyma | Apotracheal parenchyma | Apotracheal parenchyma |
| Ray | Non-storeyed, compound and multiseriate ray present, uniseriate ray scanty; Rays are homogenous and procumbent | Non-storeyed, compound and multiseriate ray present, uniseriate ray scanty; Rays are homogenous and procumbent |
| Stem wood fibre | Non-septate, non-storeyed, no pitting, large lumen. Average lumen size 19±1 µm. Narrow wall thickness, average wall thickness 5±1 µm, average fibre diameter 29±1 µm, average fibre length 692±1 µm | Non-septate, non-storeyed, no pitting, large lumen. Average lumen size 15±1 µm. Narrow wall thickness, average wall thickness 4±1 µm, average fibre diameter 24±1 µm, average fibre length 771±1 µm |
| Crystal | Crystal druses in parenchyma cells of the pith and within the phloem, one crystal druse per cell | Crystal druses in parenchyma cells of the pith and within the phloem, one crystal druse per cell |
| Starch granule | Present | Present |
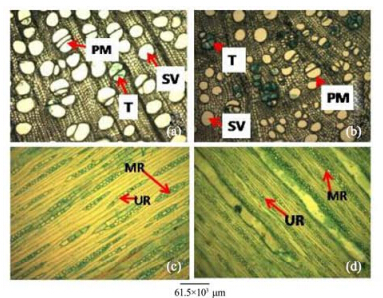 |
| Figure 4 Stem transverse sections of A. atilis(a) and A. communis(b). Stem tangential longitudinal sections of A. altilis(c) and A. communis(d). PM: Pore multiple; T: Tylose; SV: Solitary vessel; MR: Multiseriate ray; UR: Uniseriate ray |
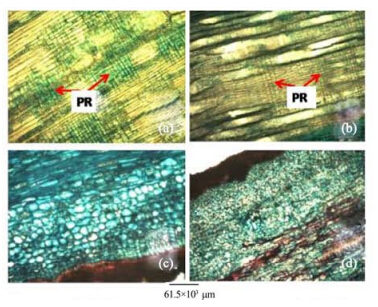 |
| Figure 5 Stem radial longitudinal sections of A. altilis(a) and A. communis(b). Stem bark longitudinal sections of A. altilis(c) and A. communis(d). PR: Procumbent ray |
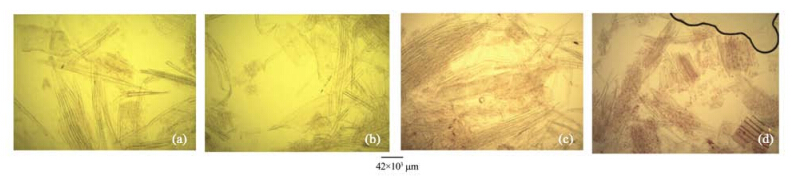 |
| Figure 6 Stem macerate of A. altilis(a, b) and A. communis(c, d) |
 |
| Figure 7 Root transverse sections of A. altilis(a, b) and A. communis(c). CD: Crystal druses; PC: Prismatic crystal |
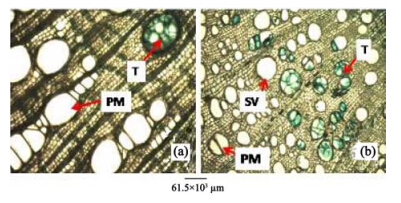 |
| Figure 8 Root transverse sections of A. altilis(a) and A. communis(b). T: Tylose; PM: Pore multiple; SV: Solitary vessel |
2)Tyloses are more frequent in A. communis than in A. altilis, while pore multiple is predominant in A. altilis in contrast to the predominance of solitary vessel in A. communis(Table 2). Just like in the stem wood fibre macerates, the root wood fibre macerate for the two species show significant differences in lumen size, wall thickness, fibre diameter and fibre length for the 2 species(Table 2, Figure 9). In cleared and peel leaves of both species the micromorphological features were the same except that the secondary veins were undulating in A. altilis but straight in A. communis(Table 4).
| Characters | A. altilis | A. communis |
| Xylem vessel | Diffuse porous system, solitary vessel, pore multiple predominant from 2-7. Tyloses less frequent | Diffuse porous system, solitary vessel predominant, pore multiple 2-7. Tyloses more frequent |
| Vessel element | Simple pitting alternate very small, perforation plate simple and scalariform, some vessels have short tail, others have long tail, some have tail at both end while some have no tail at all. Average vessel length 686±1 µm | Simple pitting, alternate very small, perforation plate simple and scalariform, some vessels have short tail while others have none; Average vessel length 184±1 µm. |
| Xylem parenchyma | Apotracheal parenchyma | Apotracheal parenchyma |
| Ray | Non-storeyed, uniseriate, multiseriate and compound ray present. Rays are homogenous and procumbent | Non-storeyed, both uniseriate and multiseriate rays present. Rays are homogenous and procumbent |
| Root wood fibre | Non-septate, non-storeyed, no pitting, large lumen. Average lumen size 30±1 µm. Narrow wall, average wall thickness 3±1 µm, average fibre diameter 36±1 µm, average fibre length 610±1 µm | Non-septate, non-storeyed, no pitting, large lumen. Average lumen size 26±1 µm. Narrow wall, average wall thickness 6±1 µm, average fibre diameter 37±1 µm, average fibre length 1, 120±1 µm |
3)The stem bark of both species were observed to have many similarities, including the presence of tannins and starch granules, while the outer bark is made up of lignified rectangular shaped dead cells, and an inner layer of thick walled parenchyma cells(Table 3). Likewise, the outer rootbark section of both species is made up of dead elongated cylindrical shaped cells, while the inner root bark layer is also made up of different shaped parenchyma cells(Table 3, Figure 10). In contrast, more trichomes were found in A. altilis stem bark than in A. communis(Table 3), while groves were found in the bark of A. communis, which were absent in A. altilis(Table 3). Furthermore, the presence of tannins and styloids only in A. communis also distinguished the two species(Table 3, Figure 10). Leaf samples in both species have thin and non-striated cuticle(Figure 11). Abaxial epidermis was thin and single layered, while the adaxial epidermis was 2-3 layered(Figure 12).
| Characters | A. altilis | A. communis |
| Stem bark | Outer bark is made up of thick lignified rectangular shaped dead cells of 8-10 layers. Inner bark is made up of wide extensive thick walled parenchyma cells of various shapes ranging from short rectangular, oval, triangular, circular to polygonal | Outer bark is made up of thick lignified rectangular shaped dead cells of 8-10 layers. Inner bark is made up of wide extensive thick walled parenchyma cells of various shapes ranging from short rectangular, oval, triangular, circular to polygonal |
| Cell inclusion | Tannin and starch granules present | Tannin and starch granules present |
| Root bark | No groove. Outer bark is made up of several layers of dead short and elongated cylindrical shaped cells. No tannin. Inner bark is made up of parenchyma cells of different shape ranges from circular, triangular, rectangular to polygonal with thick wall. Few starch granules present and few prismatic crystals present. | There were grooves on the outer bark. Outer bark is made up of several layers of dead elongated cylindrical shaped cells. Tannin present. Inner bark is made up of parenchyma cells whose shape ranges from circular, triangular, rectangular to polygonal with thick cell wall. 2-3 row of cylindrical shape parenchyma cells are parallel arranged close to the outer bark. Starch granules present in parenchyma cells. Crystal druses and styloid present. |
| Trichome | Simple unicellular short trichomes | Scarcely found |
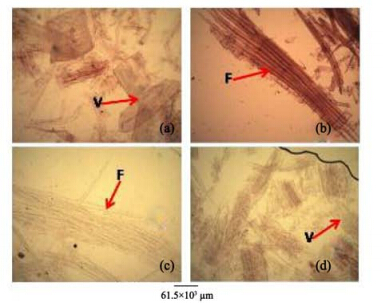 |
| Figure 9 Root macerates of A. altilis(a, b) and A. communis(c, d). F: Fibre; V: Vessel element |
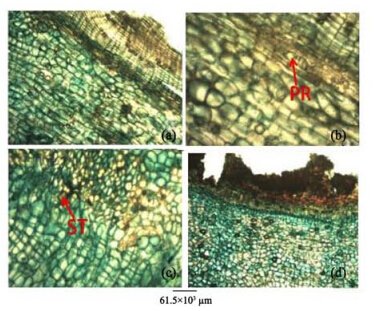 |
| Figure 10 Root bark transverse sections of A. altilis(a, b) and A. communis(c, d). PR: Prismatic crystal; ST: Styloid crystal |
| Characters | A. altilis | A. communis |
| Areole | Quadrangular, pentagonal, triangular in shape. Closed areole, no veinlet, secondary veins undulating | Quadrangular, pentagonal, triangular in shape. Closed areole, no veinlet, secondary veins straight |
| Trichome | Short simple unicellular trichome. Average length 77±1 µm. Medium simple unicellular trichome. Average length 156±1 µm. The medium size trichome have curve apex like a hook. The trichome have serrate surface | Short simple unicellular trichome. Average length 124±1 µm. Medium simple unicellular trichome. Average length 286±1 µm. Long simple unicellular trichome. Average length 770±1 µm. Some of the medium and long trichomes have curve apex like a hook. The long ones areon primary and secondary veins. The trichomes are serrated on the surface |
| Scale | Scales are mainly on the vein | Scales are numerous on the veins |
| Hair | Trichomes are on both surfaces of the leaf and along the veins, and on the leaf margin | There are trichomes on the surface of the leaf, along the vein and on the leaf margin |
| Adaxial surface | Epidermis-thick wall polygonal cells, no stomata | Epidermis-thick wall polygonal cells, no stomata |
| Abaxial surface | Epidermal cells are varied shaped polygonal cells, Steurocytic stomata present | Epidermal cells are varied shaped polygonal cells, Steurocytic stomata present |
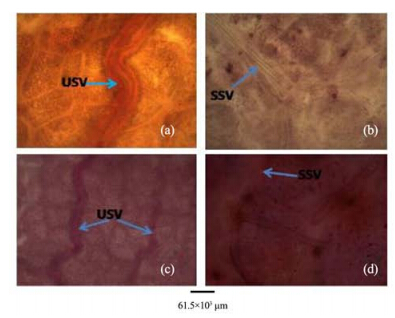 |
| Figure 11 Cleared leaves of A. altilis(a, c) and A. communis(b, d). USV: undulating secondary vein; SSV: straight secondary vein |
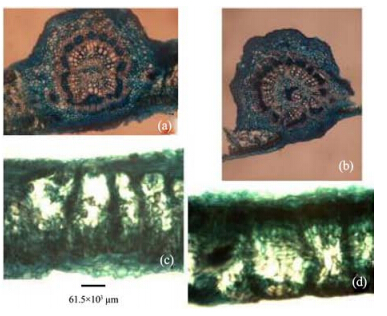 |
| Figure 12 Leaf transverse sections of A. altilis(a, c) and A. communis(b, d) |
4)Vascular bundles were closed conjoint collateral in both species, while the distribution of sclerenchyma in the vascular bundles was similar in both species(Table 5). The palisade and spongy mesophyll layers as well as crystal druses in the parenchyma cells were similar in both species. Furthermore, medullary bundles were between 1-4 in A. altilis, but up to 8 in A. communis. In addition, short cylindrical cells were predominant only in A. communis(Tables 5, 6; Figure 13). However, while both epidermal surfaces were uniseriate and thin in A. communis, similar to abaxial epidermis of A. altilis, the adaxial surface was made up of 2-3 layers of polygonal cells in A. altilis. In the transverse section of the root wood of A. altilis it was pore multiple that was predominant while solitary vessel predominate that of A. communis(Table 7). The root bark also revealed some differences. Tannin was not presented in A. altilis but was found in A. communis, few prismatic crystalis presented in A. altilis while crystal druses and styloid were found in A. communis. Few starch granules were seen in A. altilis and but numerous in A. communis(Table 8). In the cleared leaf, the secondary vein is undulating in A. altilis but straight in A. communis(Table 9).
| Characters | A. altilis | A. communis |
| Cuticle | Cuticle not prominent on both surfaces, where it is seen, it is thin and not striated | Cuticle not prominent on both surfaces, where it is seen, it is thin and not striated |
| Epidermis | Abaxial epidermis is made up of single layer(uniseriate)of thin wall short rectangular or polygonal cells. Adaxial epidermis is made up of 2 to 3 layers of thin wall polygonal cells | The epidermis on both surfaces are uniseriate thin wall, short rectangular, oval or circular shaped cells |
| Vascular bundle | Cortical bundles arranged on radii forming a broken circle, medullary bundles 1-4 at the centre. The bundles are conjoint collateral closed. Sclerenchyma cells formed a broken circle round the bundles. | Cortical bundles arranged on radii forming a broken circle, medullary bundles 1-8 at the centre. The bundles are conjoint collateral closed. Sclerenchyma cells formed a broken circle round the bundles. |
| Palisade mesophyll | Tightly packed 2-3 layers of cylindrical elongated parenchyma cells | Tightly packed 2-3 layers of cylindrical elongated parenchyma cells |
| Spongy mesophyll | Loosely packed parenchyma cells of varied shape but mostly short cylindrical cells. Idioblast present with tannin inside | Loosely packed parenchyma cells of varied shape but mostly short cylindrical cells. Idioblast present with tannin inside |
| Crystal | Crystal druses are housed in the parenchyma cell of the cortex, phloem cell, in the fibre, all in the midrib | Crystal druses are housed in the parenchyma cell of the cortex, phloem cell, in the fibre, all in the midrib |
| Characters | A. altilis | A. communis |
| Cuticle | Narrow undulating, non-striated. There are grooves on the surface | Narrow undulating, non-striated. There are grooves on the surface |
| Epidermis | 1-4 layers of varied shaped cell(short cylindrical, oval, rectangular, triangular and circular), the short cylindrical cells are arranged perpendicular to the cuticle | 1-4 layers of short cylindrical shaped cells arranged perpendicular to the cuticle. Few cells of the epidermis are either circular or oval |
| Trichomes | Simple serrated surface unicellular trichome mostly short | Simple unicellular with either straight or curve apex and serrated surface long, medium and short trichomes. More hairy than A. altilis |
| Cortex | Cortex is filled with parenchyma cells of various sizes and shapes(circular, oval, polygonal)whose size increases from epidermis to the pith. 1-3 layers of short cylindrical shaped parenchyma cells arranged parallel to the epidermal cells with some deposition of tannin. Prismatic crystals and starch granules are more concentrated within the vascular bundle | Cortex is filled with parenchyma cells of various sizes and shapes(circular, oval, polygonal)whose size increases from epidermis to the pith. 1-3 layers of short cylindrical shaped parenchyma cells arranged parallel to the epidermal cells with rich deposition of tannin. Prismatic crystals and crystal druses are presented in the cortex and more or less evenly distributed |
| Vascular bundle | The outer bundles are arranged on a radius with scattered bundles within the radius. The medullary bundles vary in number from 1 to 4 | The outer bundles were arranged on a radius with scattered bundles within the radius. The medullary bundles vary in number from 1 to 8 |
| Characters | A. altilis | A. communis |
| Root wood | Pore multiple predominant; Tylosesless frequent | Solitary vessel predominant; Tyloses more frequent |
| Stem wood macerate | ||
| Fibre lumen size | 19±1 µm | 15±1 µm |
| Fibre wall thickness | 5±1 µm | 4±1 µm |
| Fibre diameter | 29±1 µm | 24±1 µm |
| Fibre length | 692±1 µm | 771±1 µm |
| Root wood macerate | ||
| Fibre lumen size | 30±1 µm | 26±1 µm |
| Fibre wall thickness | 3±1 µm | 6±1 µm |
| Fibre diameter | 36±1 µm | 37±1 µm |
| Fibre length | 610±1 µm | 1, 120±1 µm |
| Characters | A. altilis | A. communis |
| Root bark | No tannin Few prismatic crystals Few starch granules | Tannins present Crystal druses and styloids present Numerous starch granules |
| Characters | A. altilis | A. communis |
| Vein | Secondary vein undulating | Secondary vein straight |
| Trichome | Scant trichomes and scales. No long trichome was observed | Trichome and scales are more numerous |
| Leaf epidermis | Abaxial epidermis is uniseriate while the adaxial epidermis is made up of 2-3 layers of cells | Epidermis is uniseriate on both abaxial and adaxial surfaces |
| Medullary bundle | 1-4 bundles | 1-8 bundles |
| Epidermal cell | Cylindrical shaped cells were not predominant | Mostly short cylindrical shaped cell |
5)Observations on the micromorphological characters of cuticle, epidermis, cortex and outer bundles of the vascular bundles show similarities between petioles of the two species(Table 10, Figure 14). However, in addition to the presence of prismatic crystals only in A. communis, the petiole samples from A. altilis are less hairy with short trichomes, but those of A. communis are more hairy with longer trichomes(Table 10).
| Characters | A. altilis | A. communis |
| Trichome on petiole | Less hairy with short trichome | More hairy with longer trichome |
| Petiole cortex crystal | Prismatic crystal not cited | Prismatic crystal present but very scanty |
Prominent similarities were observed in the sections of the root, stem, leaf petiole and leaf morphological character of the two species. In spite of these similarities, several differences were noted among the two species. The pores were diffuse porous system in the stem of the two species with presence of few tyloses, xylem parenchyma are apotracheal, rays are homogenous and procumbent, uniseriate ray scanty(Table 1, Figure 4). In both species, stem wood fibres are non septate, non-storeyed and no pitting with large lumen, starch granules are presented in the wood and crystal druses are also presented in the parenchyma cells of the pith and phloem(Table 1, Figure 4). However, vessel length differs in both species, being 379±1 µm in A. altilis and 184±1 µm in A. communis(Table 1). In addition, the stem wood fibre macerate revealed difference in lumen size, wall thickness, fibre diameter and fibre length between the two species(Table 1, Figure 6).
Similarly, root wood of both species revealed diffuse porous system. Simple pitting on vessel element. Xylem parenchyma is apotracheal, rays are non-storeyed, homogenous and procumbent, fibres are non-septate with large lumen(Table 2, Figure 5). However, tyloses are more frequent in A. commnis than in A. altilis, while pore multiple is predominant in A. altilis in contrast to the predominance of solitary vessels in A. communis(Table 2). In the two species, the root wood fibre macerate show significant differences in lumen size, wall thickness, fibre diameter and fibre length just as it was in the stem wood(Table 2, Figure 9). In both species, stem bark revealed some similarities, this includes presence of tannins and starch granules, outer bark is made up of lignified rectangular shaped dead cells and inner layer of thick walled parenchyma cells(Table 3). Likewise, the outer root bark sections of both species are made up of dead elongated cylindrical shaped cells, while the root bark layer are also occupied by different shaped parenchyma cells(Table 3, Figure 10). In contrast, more trichomes were found in A. altilis stem bark than A. communis(Table 3)while grooves are found on the bark of A. communis which are absent in A. altilis(Table 3), Furthermore, the presence of crystal druses and styloids only in A. communis also distinguished the two species(Table 3, Figure 10). Striking similarities were noted in the areole shapes, position of the scale, the presence and distribution of trichomes and serrated trichomes in the cleared leaves of both species(Table 3, Figure 11). Again, micromorphological studies on the leaf epidermal characters of both species indicated that the leaves were hypostomatic, with stomata present only on the abaxial surfaces, while the epidermal cells were polygonal- shaped on both leaf surfaces in the two species studied(Table 4, Figure 13). However, secondary veins were undulating in A. altilis but straight in A. communis. Furthermore, trichomes and scales were more numerous in A. communis than in A. altilis(Table 4). In the two species studied, the leaf cuticle is thin and non-striated, abaxial epidermis is a thin single layered, and whole adaxial epidermis is 2-3 layers. Vascular bundles are closed conjoint collateral in both species, while the distribution of sclerenchyma in the vascular bundles were similar in both species(Table 4). However, while both epidermal surfaces were uniseriate and thin in A. communis, similar to abaxial epidermis of A. altilis, the adaxial surface is made up of 2-3 layers of polygonal cells in A. altilis. Medullary bundles are between 1-4 in A. altilis but up to 8 in A. communis, while short cylindrical cells are predominant only A. communis(Table 5, Figures 12 and 13). The palisade and spongy mesophyll layers as well as crystal druses in the parenchyma cells are similar in both species(Table 5, Figures 12 and 13). The micromorphological characters of the cuticle, epidermis, cortex and the outer bundles of the vascular bundles revealed similarities between the petiole of the two species(Table 4, Figure 14). It was only in A. communis that prismatic crystals were sited. The petiole sample of A. altilis is less hairy with short trichomes, but those of A. communis are more hairy with longer trichomes(Tables 4 and 6). Macromorphological features of the fruits and seeds of these two Artocarpus species are taxon specific. The fruits of A. altilis have a smooth body surface and seedless while that of A. communis have spines and seeded. In fact, these are the only visible characters that distinguished these species in the field(Figures 1 and 2)for the leaves of the two species revealed mor e or less the same macromorphological features(Figure 3).
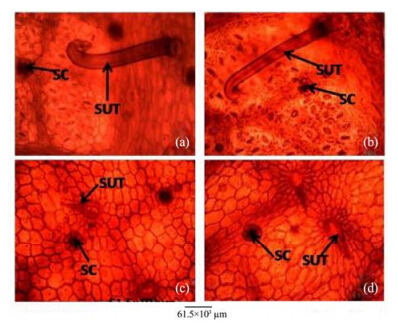 |
| Figure 13 Leaf abaxial epidermis of A. altilis(a) and A. communis(b), and leaf adaxial epidermis of A. altilis(c) and A. communis(d). SC: Scale; SUT: Simple unicellular trichome |
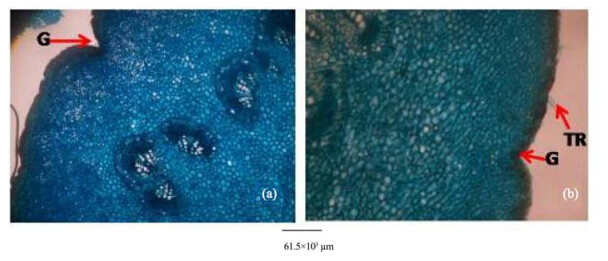 |
| Figure 14 Petiole transverse sections of A. altilis(a) and A. communis(b). G: Groove; TR: Trichome |
In addition, the stem wood of the two species revealed no visible anatomical differences(Figures 4 and 5). Also, the wood macerate of both stem and root of the two species revealed little or no anatomical differences except in the root wood fibre length(Figures 6 and 9).
The use of crystals as a diagnostic tool have been discussed by several authors(Amos, 1951; Terwelle, 1976; Illoh and Inyang, 1998). The presence of crystal druses is a common occurrence in the two species but then styloids was sited in A. communis root bark while prismatic crystals was sited in that of A. altilis(Figures 7, 10).
The anatomical study of A. altilis and A. communis revealed that both had more classificatory characters than delimiting specific character. However, there are characters that seem to be taxon specific. The study revealed that A. communis have solitary vessel predominant whereas it was pore multiple that was predominant in A. altilis at the transverse section of the root. Tyloses in vessels of the root were more frequent in A. communis than A. altilis(Figure 8).
In the midrib of the leaf at transverse plane, the medullary bundles in A. communis varied from 1-8 while 1-4 in A. altilis(Figure 12).
Furthermore in the cleared leaf, venation patterns also revealed some differences in the two species studied. While the veins of A. communis are more or less straight, that of A. altilis are undulating especially in the secondary veins(Figure 11).
In addition, prismatic crystals were found in the petiole cortex of A. communis but not seen in A. altilis. A similar observation by Akinloye et al.(2012)was used in distinguishing taxa in genus Cola. Also, starch granules were seen in the root bark of the two species but were more numerous in A. communis. A. communis has tannin in the root bark but this substance was not sited in the root bark of A. altilis.
In the cleared leaf, trichomes and scales were more abundant in A. communis than in A. altilis. This was in addition to the observation that petioles were more hairy on A. communis than on A. altilis(Figure 14). In fact, trichomes were graded as short(average length 124±1 µm), medium(average length 286±1 µm), and long(average length 770±1 µm)in A. communis. In A. altilis, only short(average length 77±1 µm) and medium(average length 156±1 µm)trichomes are presented. At transverse plane of the leaf, the adaxial and abaxial epidermis were uniseriate in A. communis but only the abaxial epidermis was uniseriate in A. altilis, and the adaxial epidermis was made up of 2-3 layers of cell.
Similarly, differences were observed in the epidermis of the two species, in terms of epidermal cell shape as well as uniseriate appearances of the epidermal surfaces. The epidermal cells in A. communis were predominantly short cylindrical shaped cell but in A. altilis cylindrical shaped cells were not predominant.
The non-prominence of the cuticle may probably be due to the fact that the plant always grows around stream/river or water logged area, therefore it does not need much of a water retention mechanism.
The taxonomic importance of morphological and anatomical characters of different plant parts for species delimitation have been reported by several authors(Metcalfe, 1968; Waly, 1999; Turki et al.,2006; Oladipo and Illoh, 2012). Waly(1999)proved that wood anatomical characters were found useful for the identification of Tamarix species. Furthermore, Oladipo and Illoh(2012)noted variations in the quantitative wood anatomical features among different Jatropha species. They concluded that wood anatomic features are taxon specific and species could be delimited by their quantitative wood characters. Metcalfe(1968)observed that anatomy of the vegetative organs could help in establishing the inter-relations of the taxa at infra- and supra-specific levels. Zaki et al.(1991)proved that the characters of the epidermal cells, the cortical zone and vascular cylinder of the young stem provided reliable characters for the distinction of closely allied species of section Tamarix. Denford(1980)reported that differences in epidermal characters of comparable organs seem always to reflect genetic differences in the plants.
Similarly, Akcin et al.(2011)reported wide variations in the anatomical structures of petioles of seven species in Lamiaceae. While leaf anatomy was reported to have no taxonomic value at generic and infrageneric levels, stem anatomy had biosystematic significance among species of the tribe Genisteae of Fabaceae in Argentina(Norverto et al.,1994). Tantawy and Naseri(2003)reported the taxonomic importance of achene surface pattern of species in Rosoideae tribe(Rosaceae)for species delimitation. Several variations and homogeneity in the micro and macro-morphological features of Hordeum species was reported by Amer et al.(2013). In the same vein, Shaheen(2007)reported diverse anatomical differences among species of Caesalpiniodeae, including vascular trace shape, pericyclic fibre forms, number of abaxial and adaxial vascular bundles, number and relative position of secondary vascular bundles, accessory vascular bundle status, the tendency of abaxial vascular bundles to divide, distribution of sclerenchyma, distribution of cluster crystals, and type of petiole trichomes, among other areas, which he concluded gave valuable data for taxonomic delimitation of the investigated species. In contrast, Sczepanik-Janyszek and Klimko(1999)reported that the observed morphological differences were not sufficient for taxonomic divisions among the studied taxa of Carex.
Likewise in this study, the variations noted in the anatomical features may not be sufficient to delimit these taxa, leaving only fruits and seed morphology as the most reliable distinguishing features between the two taxa. However, the authors suggest a comprehensive DNA analysis, as it may help provide further taxonomic data about the two species.
| Abdel-Samai MS, 2007. Characteristics of the stem-leaf transitional zone in some species of Caesalpinioideae (Leguminosae). Turkish Journal of Botany, 31: 297–310. |
| Akcin OE, Ozyurt MS, Senel G, 2011. Petiole anatomy of some Lamiaceae Taxa. Pakistan Journal of Botany, 43: 1437–1443. |
| Akinloye AJ, Illoh HC, Olagoke OA, 2012. Significant of wood anatomical features to the taxonomy of five Cola Species. Sustainable Agriculture Research, 1(2): 21–26. |
| Amer NM, Hegazy AK, Azer SA, 2013. Taxonomic revision of genus Hordeum L. (Gramineae) in Egypt. International Journal of Biodiversity and Conservation, 5(4): 198–208. |
| Amos G, 1951. Some silicious timber of British Guyana. Carribians Forest, 12: 133–137. |
| Amusa NA, Kehinde IA, Ashaye OA, 2002. Biodeterioration of breadfruit (Artocarpuscommunis) in storage and its effect on the nutrient composition. African Journal of Biotechnology, 1: 57–60. |
| Anazonwu-Bello JN, 1986. Indigenous foods and nutritional adequacy. In: Proceedings on Development of Indigenous Technology. Ministry of Science Technology, pp. 29–30. |
| Barrau J, 1976. Breadfruit and relatives. In: Simmonds NW (ed.). Evolution of Crop Plants. London, UK: Longman, pp. 201–202. |
| Bilgrami KS, Srivastava LM, Shreemali JL, 1983. Fundamentals of Botany. Shahdara, Delhi (India): Vikas Publishing House PVT Ltd.. |
| Brantjes NBM, 1981. Nectar and pollination of breadfruit: Arto-carpusaltilis (Moraceae). Acta Botanica Neerlandia, 30: 345–352. |
| Cutter EG, 1978. Plant Anatomy Part 1: Cells and Tissues (2nd ed.). London: William Clowe and Sons Ltd.. |
| Denford DKE, 1980. Flavenol glycosides and seed coat structure in certain species of Epilobiuma correlation. Experientia, 36: 299–300. |
| Esau K, 1977. Anatomy of Seed Plants (2nd ed.). New York: John Wiley and Sons. |
| Fahn A, 1977. Plant Anatomy (2nd ed.). New York: Pergamon Press. |
| Hasan SMZ, Razak AR, 1992. Parthenocarpy in seedless breadfruit (Arthocarpus incircus (Thunb.) L.). Acta Horticulturae, 321: 648–652. |
| IAWA, 1989. Hardwood features list: definitions and illustration. IAWA Bulletin, 10(3): 219–332. |
| Illoh HC, Inyang UE, 1998. Foliar Epidermis and petiole anatomy in some Nigeria Solanum Linn. Species in the Sub-Genus Leptostemanum (BITT) DUN Glinpses in Plant Research, 12: 73–86. |
| Jarrett FM, 1959a. Studies in Artocarpus and allied genera, I. General considerations. Journal of the Arnold Arboretum, 40: 1–29. |
| Jarrett FM, 1959b. Studies in Artocarpus and allied genera, III. A revision of Artocarpus subgenus Artocarpus. Journal of the Arnold Arboretum, 40: 113–155, 298–368. |
| Keay RWJ, Onochie CFA, Stanfield DFP, 1989. Trees of Nigeria. Oxford: Clarendia Press, pp. 204–205, 300. |
| Kochummen KM, 2000. Artocarpus J. R. & G. Forster, nominative conservation. In: Soepadmo E, Saw LG (eds.). Tree Flora of Sabah and Sarawak. Kuala Lumpur: Sabah Forestry Department, Forest Research Institute Malaysia, and Sarawak Forestry Department, pp. 187–212. |
| Mayaki OM, Akingbala JO, Baccus-Taylor GSH, et al., 2003. Evaluation of Breadfruit (Artocarpus communis) in traditional stiff porridge foods. Journal of Food, Agriculture and Envi-ronment, 1: 54–59. |
| Metcalfe CR, 1968. Current developments in systematic planta-natomy. In: Heywoods VH (ed.). Modern Methods in Plant Taxonomy. London, NewYork: Academic Press, pp. 45–57. |
| Metcalfe CR, Chalk L, 1989. Anatomy of the Dicotyledons (2nd ed.). Oxford: Charendon Press. |
| Norverto CA, Gonzalez-Andres F, Ortiz JM, 1994. Leaf and stem anatomy of species of Cytisophyllum, Cytisus, Chamaecytisus, Genista and Genista Sect. Teline (Fabaceae: Genisteae) as an aid for taxonomy. Israel Journal of Plant Sciences, 42: 213–225. |
| Oladipo OT, Illoh HC, 2012. Comparative wood anatomy of some members of the genus Jatropha (Euphorbiaceae) found in Nigeria. Phytologia Balcanica, 18(2): 141–147. |
| Onana J, 1995. Revue-d' Elevage-et de-medicine velerincu-ria-des-pays-tropicaus, 48: 213–219. |
| Ragone D, 2001. Chromosome numbers and pollen stainability of three species of Pacific Island Breadfruit (Artocarpus, Mora-ceae). American Journal of Botany, 88(4): 693–696. |
| Sczepanik-Janyszek M, Klimko M, 1999. Application of anatomi-cal methods in the taxonomy of Sedges (Carex L.) from section Muehlenbergianae (L. H. Bailey) Kuk. Occuring in Poland. Rocc. Akad. Rol. W. Pozaniu-cccxvi Seria Botanika, (2): 97–107. |
| Shaheen ASM, 2007. Characteristics of the Stem –Leaf Transitional zone in some Species of Caesalpinioideae (Leguminosae). Turkish Journal of Botany, 31: 297–310. |
| Tantawy ME, Naseri MM, 2003. A contribution to the achene Knowledge of Rosoideae (Rosaceae): Light Microscope and Scanning Electron Microscope. International Journal of Agri-culture and Biology, 5: 105–112. |
| Terwelle BJH, 1976. Silica grains in woody plants in neotropic especially Surinam Leiden Botanical Series. 3. Leiden Univer-sity Press, pp. 107–142. |
| Turki Z, El-Shayeb F, Shehata F, 2006. Taxonomic studies in the Camphorosmeae (Chenopodiaceae) in Egypt. 1. Subtribe Kochiinae (Bassia, Kochia and Chenolea). Flora Mediterranea, 16: 275–294. |
| Wafaa MA, Ahmad KH, Safwat AA, 2013. Taxonomic revision of genus Hordeum L. (Gramineae) in Egypt. International Journal of Biodiversity and Conservation, 5(4): 198–208. |
| Waly NM, 1999. Wood anatomical characters of the Egyptian Tamarix L. species and its taxonomic significance. Taeckhol-mia, 19(2): 115–125. |
| Zaki MA, Hosni HA, Araffa S, 1991. Morphological and anatom-ical features of species of Tamarix in Egypt. Journal of Applied Science, 6(10): 502–511. |
| Zerega NJC, Ragone D, Motley TJ, 2005. Systematics and species limits of breadfruit (Artocarpus, Moraceae). Systematic Botany, 30(3): 603–615. |
 2015, 7
2015, 7


