Article Information
- ManXiao Zhang, HuiJuan Pei, YouFu Zhang, Tuo Chen, GuangXiu Liu. 2015.
- Foliar carbohydrate differs between Picea crassifolia and Sabina przewalskii with the altitudinal variation of Qilian Mountains, China
- Sciences in Cold and Arid Regions, 7(2): 180-188
- http://dx.doi.org/10.3724/SP.J.1226.2015.00180
Article History
- Received: July 25, 2014
- Accepted: November 21, 2014
2. Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730060, China;
3. Henan University of Science and Technology, Luoyang, Henan 471003, China
1 Introduction
NSC metabolism is affected by plant hereditary properties and environmental stress(drought, low temperature, UV-B radiation). NSC concentration is used as a measurement in the relationship between plant carbon absorbance(photosynthesis assimilation) and carbon consumption(growth and respiration)(Li MH et al., 2008), and NSCs were key responses to drought, playing an important role in plant mortality or widespread die-off(McDowell, 2011). At the same time, NSC metabolisms strongly influence the growth and development of plants in turn(Li MH et al., 2008, 2012). For example, the biosynthesis of cellulose and lignin depends largely on NSC supply. Plants synthesize cellulose and lignin when conditions are optimal and NSC is not limiting(Genet et al., 2011; Richet et al., 2012).
Cellulose comprises 40%-50% woody dry matter, with lignin the second most abundant component of wood after cellulose, accounting for 15%-35% of woody dry matter(Donaldson, 2001; Delmer and Haigler, 2002). Cellulose and lignin are the main components of cell walls, constituting the major SC and a strong C sink within the plant(Richet et al., 2011). Cellulose and lignin concentrations change significantly under various environmental stresses, but these compounds are essential for plant biomass, defense, growth and development(Richardson, 2004; Moura et al., 2010; Genet et al., 2011; Cesarino et al., 2012; Richet et al., 2012).
The photosynthetic capacity of plants is closely related with tissue N concentration, where 75% of plant N is concentrated in the chloroplast, most of which is used in the construction of photosynthetic structures. Thus, N plays a key role in the metabolism of photosynthesis and plant growth(Evans, 1989). Leaf N concentration has been considered as a comprehensive index to assess plant survival and acclimatization during the evolutionary process(Reich et al., 2003).
Various environmental factors change with altitudinal variation on the Qilian Mountains, such as temperature, humidity and light. The plant morphous, structure, physiological and biochemical domains show adaptive change with elevation. Recent studies investigated the antioxidative systems of S. przewalskii and P. crassifolia(Chen et al., 2006; Zhang et al., 2009) and foliar δ13C changes(Zhang et al., 2010)in these two evergreen species in their response to altitudinal variation. Nevertheless, there has been no examination on how NSC, SC and N concentrations in the leaves of S. przewalskii and P. crassifolia varied with changes in altitude. The status of foliar NSC, SC among the two alpine species at different tree lines in the same area during the growing season was never examined(Li MC et al., 2008; Zhang et al., 2009; Zhang et al., 2010; McDowell et al., 2011; Anderegg et al., 2012; Fajardo et al., 2012; Hoch and Körner, 2012; Zhu et al., 2012).
The two species, P. crassifolia and S. przewalskii, provide a typical model for comparing leaf ecophysiological response to environment stress across an altitudinal gradient. Qinghai spruce(P. crassifolia)accounts for over 90% of the trees in the Qilian Mountains, occurring from 2, 500 to 3, 500 m(tree line) and mainly inhabits shady or semi-shady slopes. The mean annual temperature across this gradient is about 4-12 °C. On the steep northeastern slopes of the QTP(Qinghai-Tibet Plateau), Qinghai spruce form the dominant forest st and s to the tree line at approximate 3, 500 m. At higher altitudes, Potentilla shrubs replace this spruce species. S. przewalskii, a protected species in China, is distributed across the northern part of Sichuan Province; in the southern and eastern parts of Qinghai Province; in southern and central parts of Gansu Province; from 2, 600 to 4, 300 m(tree-line)on subalpine and alpine areas of the Qinghai-Tibet Plateau. The annual mean temperature is approximately 0.5 °C, resulting in this species being part of a freeze-resistant coniferous forest. These species constitute a unique sub-alpine dark coniferous forest belt on full-sun and half-sun hillsides on small plateaus, establishing the tree line around 4, 300 m in the Qilian Mountains.
Research that examined mobile carbohydrates in tree-line species found no consistent evidence for C limitation of growth(Li MH et al., 2008; McDowell et al., 2011; Anderegg et al., 2012; Zhu et al., 2012). Zhu et al.(2012) reported that NSC levels in plant tissue are lowest at the beginning of the growing season but plants grown at their altitudinal limit do not show lower NSC concentrations compared with plants at lower elevations. Hoch and Körner(2012) thought that NSC concentrations were not depleted across any altitudinal gradient and the increasing trend(primarily because of higher starch concentrations rather than free sugars)of NSC concentrations with altitude supports the growth-limitation hypothesis. Fajardo et al.(2012) demonstrated no reduction in NSC at the tree line, with NSC increasing in most species(each represented by one common population)towards their upper altitudinal limit. The disparity between C acquisition and SC investment at low temperature(accumulation of NSC)occurs among genotypes not adapted to tree-line environments(Fajardo et al., 2012). Increases in NSC reserves are expected if growth declines faster than photosynthesis(McDowell et al., 2011) and changes in stored carbohydrate concentrations in plants indicate a supply status of carbohydrates(Li et al., 2002). Anderegg et al.(2012) showed a growth decline and increased C allocation to roots. Some researchers have suggested that the active loading of phloem with NSCs allows plants to maintain low foliar concentrations of NSCs, leading to a substantial increase in growth potential(Turgeon, 2010).
Our objective was to examine the leaves of S. przewalskii and P. crassifolia to elucidate the response of C, N, NSC and SC concentrations to altitudinal variation during the main growth-period. We desire to examine which hypothesis, the carbon limitation or the growth limitation, best fits the plant response between C, N, NSC, SC and altitude. This will provide evidence to further explain if S. przewalskii and P. crassifolia tree-line formation is correlated with tissue NSC concentrations along an altitudinal gradient.
2 Materials and methods 2.1 Overview of investigation areaThe investigation area is located at the SiDalong forest(38°25.257′N-38°26.952′N; 99°53.929′E- 99°56.595′E), Sunan County, Zhangye, Gansu Province, China. The altitudinal range is 2, 600-3, 400 m, with a mean annual temperature of 0.7 °C. The mean temperature in January is −13.3 °C and the mean temperature in July is 11.8 °C. Mean annual precipitation is 433.6 mm, with most(89.2%)falling between May and September. Mean annual evaporation capacity is 1, 061.8 mm, with a mean annual relative humidity of 60%. The temperature lapse-rate is 0.58 °C/100 m and the precipitation rate is 18.6 mm/100 m. Thus, with increasing altitude the temperature, evapotranspiration and O2 or CO2concentration decline, while precipitation and radiation increase(Xu et al., 2009). The soil type at the forest site is Huihe soil.
Weather data at three elevations representing the lower(2, 700 m), middle(3, 100 m) and higher(3, 400 m)areas were collected from our measurements located within the SiDalong forest region. Mean growing temperatures(the beginning of August)for the lower, middle and higher elevation areas are 15.7, 12.1 and 8.56 °C, respectively. The corresponding mean rainfall values are 282, 379 and 393 mm, with evapotranspiration rates of 1, 162, 975 and 859 mm, respectively. These values indicate that the altitudinal gradient studied is linked to differences in temperature and variations in precipitation and evapotranspiration.
2.2 SamplingSampling sites were established on the sunny(southern) and shady slopes(northern)across the complete altitudinal range(2, 700-3, 400 m). The slope at the sites ranged from 25% to 50%, with leaf samples taken at 2, 700, 2, 800, 2, 900, 3, 000, 3, 100, 3, 200, 3, 300 and 3, 400 m. S. przewalskii and P. crassifolia above 3 m tall and aged approximately 35-40 years were selected for sampling at each site(total 16 sites)across each 800 m 'step'. One-year-old needles were r and omly collected from the southern side of each tree crown and the upper-third of the canopy from 10 trees with a pruning pole between 12:00 and 14:00 every day at the beginning of August in 2011(the mid-stage of the growing season or fast growing period)at each site. Each sample was placed in liquid N2 and stored at −80 °C for the NSC, SC, C and N concentration analysis.
2.3 Assay methodsThe determination of soluble sugars(sucrose and fructose) and starch concentration in leaves were assayed according to the methods reported by Yoshida et al.(1976), Brooks et al.(1986), Zhao et al.(2000) and Chen et al.(2012). The concentration determination of SC(cellulose and lignin)in leaves was performed following the Biqinluoke method(1981, translated by Jing JH and Ding ZR, 1987). The concentration of carbon and nitrogen were assayed using an elemental analyzer at the Analysis and Determination Centre, Lanzhou University(Vario EL).
The concentration of soluble sugars, starch, cellulose, lignin and C(Cmass) and N(Nmass)concentration were expressed on a dry weight basis(concentration mg/g DW or concentration % DW).
2.4 Statistical methodsTo calculate the NSC concentration we used the following equation: NSC concentration = soluble sugar concentration + starch concentration. The influences of elevation and species were analyzed with a two-way ANOVA, with elevation and species as fixed factors to determine elevational trends in concentrations of foliar NSC and its components for overall(the two treeline cases combined)SC, N, and C at each sample plot. At the SC level for each species, two-way ANOVAs were repeatedly used with elevation and foliar N concentration as factors to examine some interaction effect between altitude, N, C and NSC concentration. Altitudinal gradients do affect plant's growth; therefore we would suspect that altitude will affect total plant carbohydrates. Differences among elevations were compared and tested for significance at the p <0.05 level using a Tukey-Kramer honest significant difference(HSD)test. All statistical tests were performed with JMP 8.0(SAS Institute, Cary, NC, USA), p ≤0.01 was considered as a strong significant difference. Excel 2007 was used for all figures.
3 Results 3.1 Change of NSC concentration in leaves of S. przewalskii and P. crassifolia at different altitudesThe soluble sugar concentration in P. crassifolia leaves was significantly higher(p <0.01)than that in S. przewalskii leaves at the same altitude(2, 700-3, 400 m)(Figure 1a). There was an increase in foliar soluble sugar concentration with increasing elevation at the lower sampling area(2, 700-3, 100 m)in P. crassifolia, and then decreased at the higher altitude(3, 100-3, 400 m). Conversely, S. przewalskii showed a fall-rise change in soluble sugars at this altitude(p <0.05).
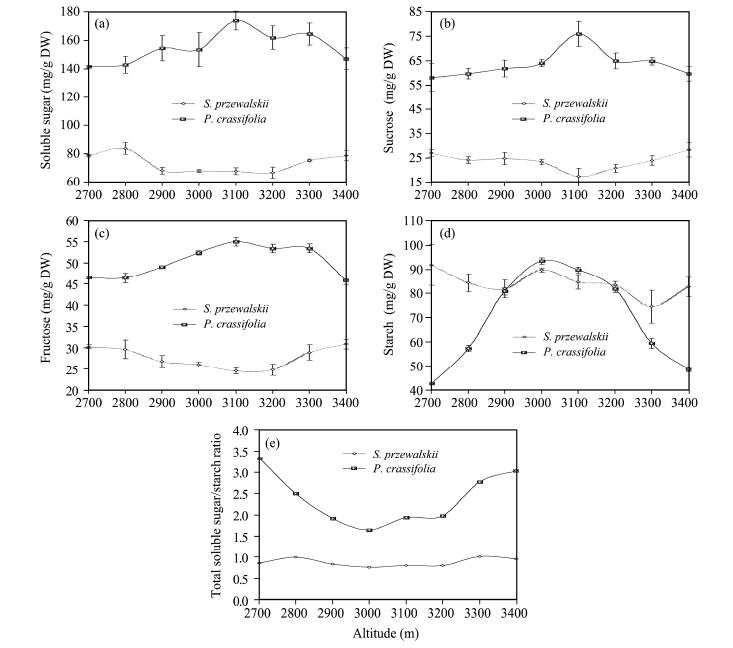
|
| Figure 1 Nonstructural carbohydrates concentration and total soluble sugar/starch concentration ratio in leaves of Sabina przewalskii and Picea crassifolia growing at different altitudes. Sampling sites were on sunny and shady slopes ranging from 2, 700 to 3, 400 m. We selected samples from trees that were 3 m tall and 35-40 years-old. One-year-old needles were r and omly collected from the southern side of each tree crown and the upper-third of the canopies from 10 trees. Samples were acquired with a pruning pole between 12:00-14:00 every day at the beginning of August(the mid stage of growth season or fast growing period)in 2011. Each point represents the average nonstructural carbohydrates concentration of 3 true replicates, each replicate consists of 300 g of needles from 10 different plants(30 g needles/tree)respectively in each sampling site. Values are the means of three replicates±s.e.(s.e. is st and ard error, represented with bar) |
In P. crassifolia and S. przewalskii leaves, sucrose and fructose concentrations showed a similar change to soluble sugar concentration with altitude(Figures 1b and 1c), but the starch concentration(Figure 1d)in P. crassifolia leaves significantly increased with elevation(2, 700-3, 000 m; p <0.01). Starch concentrations were highest at 3, 000 m, declining sharply between 3, 000 and 3, 400 m(p <0.001)returning to a similar pattern observed in other NSCs(p <0.01)(Figures 1a-1d). However in S. przewalskii, foliar starch concentrations showed no significant discrepancies or fluctuations with increasing altitude(p >0.05). The mean starch concentration in S. przewalskii leaves was 0.216 times higher than that in P. crassifolia leaves. The ratio of total soluble sugar with starch concentration for P. crassifolia decreased with increasing altitude in the low altitude area, reaching the lowest value at 3, 100 m, then showed a positive association with increasing altitude. In contrast, S. przewalskii showed no significant relationship. The sugar/starch concentration ratio was significantly higher in P. crassifolia(p <0.01)than in S. przewalskii(Figure 1e).
3.2 Change of SC(cellulose and lignin)concentrations in S. przewalskii and P. crassifolia leaves at different altitudesThe mean SC(cellulose and lignin)concentration in S. przewalskii leaves was 0.240-0.099 times higher(cellulose and lignin, respectively)than that in P. crassifolia leaves(p <0.01; Figures 2a-2b). We also observed a significant altitudinal trend. The concentration of cellulose in S. przewalskii increased with elevation(p <0.05)at the 2, 700-3, 100 m level, then decreased at the 3, 100-3, 400 m level(p <0.01). The cellulose content of P. crassifolia leaves declined at the 2, 700-3, 100 m level, increasing at the 3, 100-3, 400 m level. Lignin concentrations in S. przewalskii and P. crassifolia leaves slowly declined at the 2, 700-3, 400 m level(p <0.01).
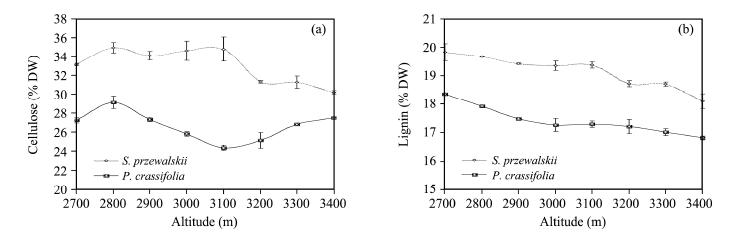
|
| Figure 2 Structural carbohydrates content in leaves of S. przewalskii and P. crassifolia at different altitudes(Sampling see Figure 1 legend for the structural carbohydrates concentration analysis). Each point represents the average content of 3 true replicates, each replicate consists of 300 g of needles from 10 different plants(30 g needles/tree)respectively in each sampling site. Values are the means of three replicates±s.e.(s.e. is st and ard error, represented with bar) |
We found no significant changes in total carbon content(p >0.05, Figure 3a)but the nitrogen concentrations tended to decrease(p <0.01, Figure 3b)for S. przewalskii and P. crassifolia leaves with elevation. At the same time, total carbon and nitrogen concentrations were higher in S. przewalskii than in P. crassifolia(p <0.01)with the C/N ratio significantly less in S. przewalskii than in P. crassifolia(p <0.0001, Figure 3c). Furthermore, changes in SC concentrations showed altitudinal trend, and also showed a positive linear correlation to N concentration in S. przewalskii leaves(Table 1).
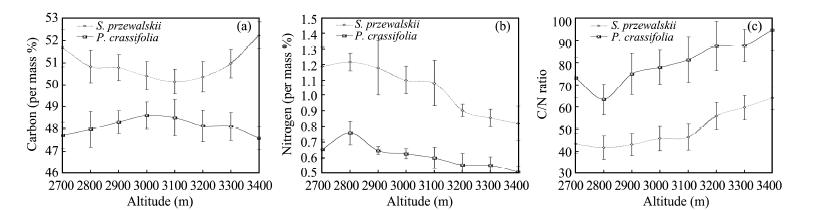
|
| Figure 3 Carbon(a), nitrogen(b)concentration and C/N ratio(c)in leaves of S. przewalskii and P. crassifolia at different altitudes(Sampling see Figure 1 legend for the Carbon, Nitrogen concentration and C/N ratio analysis). Each point represents the average C, N concentration and C/N ratio of 3 true replicates, each replicate consists of 300 g of needles from 10 different plants(30 g needles/tree)respectively in each sampling site. Values are the means of three replicates±s.e.(s.e. is st and ard error, represented with bar) |
| Y | a | b | Correlation coefficient, r |
| Lignin concentration(S. przewalskii) | 15.525 | 3.4744*** | 0.9140*** |
| Cellulose concentration(S. przewalskii) | 22.286 | 10.3260** | 0.7794** |
| Lignin concentration(P. crassifolia) | 14.412 | 4.9337** | 0.6160** |
| Cellulose concentration(P. crassifolia) | 20.711 | 9.7420 | 0.2611 |
| Y=a+bX; Y=SC concentration(%DW), X=N concentration(per mass, %)respectively at 8 elevation; the number of samples =192; **, p <0.01; ***, p <0.001. | |||
S. przewalskiishowed specific upper altitudinal limits(tree line at about 4, 300 m), with lower temperature-adapted alpine life-form and less total sugar concentration in the leaves(Figures 1a-1c)than P. crassifolia(tree-line at about 3, 500 m). Therefore in plants, a C supply limitation(Stevens and Fox, 1991; Wardle, 1993)is an unlikely cause for low temperature range limits. The soluble sugar surplus in P. crassifolia resulted from altitudinal stress leading to greater reduction in growth compared with photosynthesis, and greater reduction in photosynthesis compared with maintenance respiration.
From an evolutionary perspective, S. przewalskii maintains lower foliar concentrations of total soluble sugar(NSC)for economic consideration of inventory costs. This indicates that maintaining lower soluble sugar levels and a greater relative abundance of starch(reserve status of NSC)in leaves improves return on investment in carbohydrate synthesis, leading to a substantial increase in growth and survival potential(Figures 1d and 1e; Turgeon, 2010; McDowell, 2011; McDowell et al., 2011; Richet et al., 2011, 2012; Cesarino et al., 2012; Chen et al., 2012). Therefore, in low temperature-adapted plants, light-saturated photosynthesis is considerably less sensitive to temperature than growth. Consequently, it is possible that high-altitude tree-line species have been selected as the genotypes(because of genetic adaptation)of reduced SG accumulation.
This evolutionary trait occurred because of consumption of the growth or regrowth to SG and the need to reserve starch of the NSC, where surplus SG(after meeting consumption of the growth)was converted into starch during the growing season leading to greater reduction in SG concentration compared with the lower-altitude tree-line species(Fajardo et al., 2012). In many tree species, these reserve functions are mainly carried out by starch, which is degraded to soluble carbohydrates during the dormant season to maintain active respiration and provide protection against freezing(Chen et al., 2012). This suggests an ecophysiological significance of reserve status accumulation of NSC during the growing season in determining the winter survival and spring re-growth of plants at the upper altitudinal limit or in an adverse environment(Zhu et al., 2012). However, the mechanisms regulating and controlling the altitudinal limits of tree species unable to reach the tree-limit are poorly understood(Mellert et al., 2011; Rand in et al., 2013).
4.2 Higher total C, N and SC contents in the leaves of high-elevation tree-line species indicating better assimilation strategies under environmental stressPlant C exists in various compounds including SG, starch, cellulose and lignin(Kogami et al., 2001). N concentration in leaves belongs to the structural characteristics of leaves, with N concentrations in different plant species differing with changing altitude(Poorter and Evans, 1998). We found that total C, including SC and N concentrations in S. przewalskii leaves, was higher than in P. crassifolia(Figures 2 and 3, p <0.01). These results suggest higher photosynthetic capabilities(i.e., increasing total C content)can prompt higher accumulation of leaf N concentration among different species(Osmond et al., 2009). The increase of leaf N concentration can add N to non-photosynthetic tissue or organs in leaves, improving cellular osmotic pressure and strengthening the protection of water in vivo(Meziane and Shipley, 2001). This suggests that S. przewalskii had strong assimilation processes for C, including relatively better resistance to adverse environmental conditions compared with P. crassifolia.
In this study, the N concentration in S. przewalskii and P. crassifolia leaves tended to decrease and total C concentration showed no significant change, but the C/N ratio increased with altitude(2, 700-3, 400 m; Figures 3a-3c; p <0.01). Concurrently, the C/N ratio in S. przewalskii leaves was significantly less than in P. crassifolia. The changes in SC concentrations showed altitudinal trend, and also showed a positive linear correlation to N concentration in S. przewalskii leaves(Figures 2 and 3, p <0.01). P. crassifolia leaves showed no significant correlation with SC concentrations. This implies that differences occur between the two species to changes in climate and the different nutrient allocation strategy in different species(Wright et al., 2001)with altitude.
These results indicate that higher tree-lines species have a stronger resistance with increasing total C and N content in S. przewalskii leaves(Xu and Zhou, 2007), demonstrating that assimilation of C and N into the SC or existing components of living tissue in S. przewalskii should be significantly potent as compared with P. crassifolia under adverse conditions. It is possible that N was involved in the SC biosynthesis in S. przewalskii leaves(Table 1). The adaptive advantage of active phloem loading to sink organs for increasing plant growth or regrowth and survival potential in S. przewalskii may explain the negative relationship between S. przewalskii and P. crassifolia foliar total C concentration with increasing altitude(Turgeon, 2010; Zhang et al., 2010).
C concentration showed no significant change with increasing altitude(p >0.05), while N concentration showed a negative relationship with altitude, resulting in an increased foliar C/N ratio for P. crassifolia and S. przewalskii with increasing altitude(Figure 3, p <0.01). If the assimilation ofS. przewalskii is stronger, then foliar C/N ratio would be lower than that in P. crassifolia. Thus, the foliar C/N ratio showed a negative correlation with the assimilation in alpine plants. This suggests that the foliar C/N ratio was increasing while the assimilation was decreasing with altitude(Figures 1-3, p <0.01).
4.3 The altitudinal trend of foliar NSC concentrations following the principle of the GLH or CLH depend on acclimation of the different alpine life-form to the environmentWhen assimilated C is limited, evolutionarily successful plants must partition C preferentially towards survival or NSC reserves and not towards structural growth such as cellulose or lignin synthesis(Moura et al., 2010; Richet et al., 2011, 2012; Anderegg, 2012; Cesarino et al., 2012) and N availability(Poorter and Evans, 1998; Pensa and Sellin, 2002; Vosa et al., 2005; Lemairea et al., 2007). This leads to considerable redirection of the carbon skeletons(Richardson, 2004; Genet et al., 2011). In P. crassifolia, foliar NSC increased(Figures 1a-1d, 3a, p <0.05)but SC and total N levels decreased(Figures 2a and 2b)at lower altitudes(2, 700-3, 100 m). This suggests that mobile carbon levels were in excess of growth requirements because of the readjustment to the structural growth with increasing altitude, adhering to the principle of the growth-limitation hypothesis(GLH). The NSC concentration slowly declined from 3, 100 to 3, 400 m(near tree-line)as the carbon supply was insufficient from the shortage of photo-assimilates caused by low temperatures and a relatively short growing season, i.e., the carbon limitation hypothesis(CLH)(Stevens and Fox, 1991; Körner, 1998, 2007; Kogami et al., 2001; Hoch et al., 2002; Li and Kruchi, 2005; Piper et al., 2006; Richet et al., 2011, 2012).
This result may have occurred primarily from the strong fluctuation in starch concentrations(p <0.001)rather than free sugars(Figures 1d and 1e, p <0.05)with relatively harsh conditions at the altitudinal limit(Figures 1a-1d, p <0.05). This result is consistent with a recent hypothesis for the alpine tree-line formation(Susiluoto et al., 2010; Genet et al., 2011), but inconsistent with excess C in the higher altitude tree st and s(Fajardo et al., 2012; Hoch and Körner, 2012). Our study provided some evidence of C shortages with the decline of NSC, total C, N and lignin levels in P. crassifolia leaves(low-elevation tree-line species)at 3, 100-3, 400 m(Figures 1-3). Furthermore, we found no significant correlation(p >0.05)between foliar N and SC content for P. crassifolia, but cellulose concentrations increased near the tree-line(3, 100-3, 400 m). This indicates that leaf C/N metabolism of P. crassifolia did not contribute to structural growth but maintained the biomass recalcitrance of the species own survival under harsh growth conditions(Poorter and Evans, 1998; Pensa and Sellin, 2002; Richardson, 2004; Vosa et al., 2005; Lemairea et al., 2007; Genet et al., 2011).
In contrast, NSC levels in leaves of the high-elevation tree-line species, i.e., S. przewalskii, declined but SC and N concentrations showed no significant change at 2, 700-3, 100 m(Figures 1-3, p >0.05), while at 3, 100 to 3, 400 m, NSC increased, with SC and N concentrations decreased(Figures 1-3, p <0.05). Moreover, the altitudinal trends of starch and the total carbon or the sugar/starch ratio in S. przewalskii leaves was non-significant with increasing altitude(Figures 1d and 1e, p >0.05). These results suggest that the NSC content in S. przewalskii leaves was sufficient to maintain structural leaf growth, while decreasing NSC levels with increasing elevation resulted from the adaptive advantage of active phloem loading to sink organs. This resulted in increased growth-potential under more favorable conditions at lower altitudes(Turgeon, 2010). Alternatively, under unfavorable conditions at high altitudes(3, 100-3, 400 m), the altitudinal trend of total C, NSC and N, SC content in species at the high-elevation tree-line followed the GLH. These results were completely different from the altitudinal changes in the low-elevation tree-line species, i.e., P. crassifolia. Hence, tissue NSC concentrations along the altitudinal gradient varied significantly with acclimation of different tree species to altitude, reflecting the genotype-dependent C balance. This suggests that the variation in some ecophysiological traits of NSC concentrations, along an altitudinal gradient is controlled genetically(Vitasse et al., 2009).
5 ConclusionResearch examining mobile carbohydrates in tree-line species, found no consistent evidence for carbon limitation of growth. This is the first paper which compared the foliar carbohydrate difference between Picea crassifolia and Sabina przewalskii with altitudinal variation in the Qilian Mountains. Experimental data analysis in the field showed that foliar less soluble sugar and more starch content enabled high-elevation tree-line species to have a strong acclimation to adverse environments for survival and growth/regrowth. Foliar higher total C, N and SC contents of high-elevation tree-line species indicate better assimilation strategies under environmental stress. The trend of foliar NSC concentrations along an altitudinal gradient follows the principle of the growth-limitation hypothesis(GLH)or carbon limitation hypothesis(CLH), which depends on the acclimation of different alpine life-forms to the environment. This suggests that the variation in some ecophysiological traits of foliar NSC, C, N and SC concentrations along an altitudinal gradient is both regulated environmentally and controlled genetically.
Acknowledgments: This work was supported by The National Science Foundation of China(Nos. 31160086, 31200299).| Anderegg WRL, 2012. Complex aspen forest carbon and root dynamics during drought. Climatic Change, 111(3): 983-991. DOI: 10.1007/s10584-012-0421-9. |
| Anderegg WRL, Berry JA, Smith DD, et al., 2012. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proceedings of the National Academy of Sciences of the United States of America, 109(1): 233-237. DOI: 10.1073/pnas.1107891109. |
| Biqinluoke XH (Translated by Jing JH, Ding ZR), 1987. Analysis Methods of Plant Biochemistry. Beijing: Science Press. |
| Brooks JR, Griffin VK, Kattan MW, 1986. A modified method for total carbohydrate analysis of glucose syrups and other starch hydrolysis products. Cereal Chemistry, 63: 465-466. |
| Cesarino I, Araújo P, Domingues Júnior AP, et al., 2012. An overview of lignin metabolism and its effect on biomass recalcitrance. Brazilian Journal of Botany, 35(4): 303-311. |
| Chen T, Pei HJ, Zhang YF, et al., 2012. Seasonal changes in non-structural carbohydrates and sucrose metabolism enzymes in two Sabina species. Acta Physiologiae Plantarum, 34(1): 173-180. DOI: 10.1007/s11738-011-0815-8. |
| Chen YP, Zhang MX, Chen T, et al., 2006. The relationship between seasonal changes in anti-oxidative system and freezing tolerance in the leaves of evergreen woody plants of Sabina. South African Journal of Botany, 72(2): 272-279. DOI: 10.1016/j.sajb.2005.09.004. |
| Delmer DP, Haigler CH, 2002. The regulation of metabolic flux to cellulose, a major sink for carbon in plants. Metabolic Engineering, 4(1): 22-28. DOI: 10.1006/mben.2001.0206. |
| Donaldson LA, 2001. Lignification and lignin topochemistry—an ultrastructural view. Phytochemistry, 57(6): 859-873. DOI: 10.1016/S0031-9422(01)00049-8. |
| Evans JR, 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia, 78: 9-19. DOI: 10.1007/BF00377192. |
| Fajardo A, Piper FI, Pfund L, et al., 2012. Variation of mobile carbon reserves in trees at the alpine treeline ecotone is under environmental control. New Phytologist, 195: 794-802. DOI: 10.1111/j.1469-8137.2012.04214.x. |
| Genet M, Li MC, Luo TX, et al., 2011. Linking carbon supply to root cell-wall chemistry and mechanics at high altitudes in Abies georgei. Annals of Botany, 107: 311-320. DOI: 10.1093/aob/mcq237. |
| Hoch G, Körner C, 2012. Global patterns of mobile carbon stores in trees at the high-elevation tree line. Global Ecology and Biogeography, 21: 861-871. DOI: 10.1111/j.1466-8238.2011. 00731.x. |
| Hoch G, Popp M, Körner C, 2002. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. OIKOS, 98(3): 361-374. DOI: 10.1034/j.1600-0706.2002.980301.x. |
| Kogami H, Hanba YT, Kibe T, et al., 2001. CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum leaves from low and high altitudes. Plant, Cell and Environment, 24(5): 529-538. DOI: 10.1046/ j.1365-3040.2001.00696.x. |
| Körner C, 1998. A reassessment of high elevation treeline positions and their explanation. Oecologia, 115(4): 445-459. DOI: 10.1007/s004420050540. |
| Körner C, 2007. The use of 'altitude' in ecological research. TRENDS in Ecology and Evolution, 22: 569-574. DOI: 10.1016/j.tree.2007.09.006. |
| Lemairea G, van Oosteromb E, Sheehy J, et al., 2007. Is crop N demand more closely related to dry matter accumulation or leaf area expansion during vegetative growth? Field Crops Research, 100(1): 91-106. DOI: 10.1016/j.fcr.2006.05.009. |
| Li MC, Luo TX, Zhu JJ, et al., 2008. Advances in formation mechanism of alpine timberline and associated physioecological characteristics of plants. Acta Ecologica Sinica, 28: 5583-5591. |
| Li MH, Cherubini P, Dobbertin M, et al., 2012. Responses of leaf nitrogen and mobile carbohydrates in different Quercus species provenances to moderate climate changes. Plant Biology, 15(Suppl. 1): 174-188. DOI: 10.1111/j.1438-8677.2012. 00579.x. |
| Li MH, Hoch G, Körner C, 2002. Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees, 16: 331-337. DOI: 10.1007/s00468-002-0172-8. |
| Li MH, Kruchi R, 2005. The state of knowledge on alpine treeline and suggestions for future research. Journal of Sichuan Forestry Science and Technology, 26(4): 36-42. |
| Li MH, Xiao WF, Shi PL, et al., 2008. Nitrogen and carbon source-sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant, Cell and Environment, 31(10): 1377-1387. DOI: 10.1111/j.1365-3040.2008.01848.x. |
| McDowell NG, 2011. Mechanisms linking drought, hydraulics, carbon metabolism and vegetation mortality. Plant Physiology, 155(3): 1051-1059. DOI: 10.1104/pp.110.170704. |
| McDowell NG, Beerling DJ, Breashers DD, et al., 2011. Interrelated mechanisms of drought-induced tree mortality. Trends in Ecology & Evolution, 26: 523-531. |
| Mellert KH, Fensterer V, Küchenhoff H, et al., 2011. Hypothesis-driven species distribution models for tree species in the Bavarian Alps. Journal of Vegetation Science, 22(4): 635-646. DOI: 10.1111/j.1654-1103.2011.01274.x. |
| Meziane D, Shipley B, 2001. Direct and indirect relationships between specific leaf area, leaf nitrogen and leaf gas exchange. Effects of irradiance and nutrient supply. Annals of Botany, 88(5): 915-927. DOI: 10.1006/anbo.2001.1536. |
| Moura JC, Bonine CA, de Oliveira Fernandes Viana J, et al., 2010. Abiotic and biotic stresses and changes in the lignin content and composition in plants. Journal of Integrative Plant Biology, 52(4): 360-376. DOI: 10.1111/j.1744-7909.2010.00892.x. |
| Osmond CB, Austin MP, Berry JA, et al., 2009. Responses of growth and ecophsiology of plants to altitude. Ecology and Environmental Sciences, 18: 722-730. |
| Pensa M, Sellin A, 2002. Needle longevity of Scots pine in relation to foliar nitrogen content, specific leaf area, and shoot growth in different forest types. Canadian Journal of Forest Research, 32(7): 1225-1231. DOI: 10.1139/x02-044. |
| Piper FI, Cavieres LA, Reyes-Díaz M, et al., 2006. Carbon sink limitation and frost tolerance control performance of the tree Kageneckia angustifolia D. Don (Rosaceae) at the treeline in central Chile. Plant Ecology, 185(1): 29-39. DOI: 10.1007/s11258-005-9081-4. |
| Poorter H, Evans JR, 1998. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia, 166(1-2): 26-37. DOI: 10.1007/s004420050560. |
| Randin CF, Paulsen J, Vitasse Y, et al., 2013. Do the elevational limits of deciduous tree species match their thermal latitudinal limits? Global Ecology and Biogeography, 22(8): 913-923. DOI: 10.1111/geb.12040. |
| Reich PB, Wright IJ, Cavender-Bares J, et al., 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences, 164(Suppl. 3): S143-S164. DOI: 10.1086/374368. |
| Richardson AD, 2004. Foliar chemistry of balsam fir and red spruce in relation to elevation and the canopy light gradient in the mountains of the northeastern United States. Plant Soil, 260: 291-299. |
| Richet N, Afif D, Huber F, et al., 2011. Cellulose and lignin biosynthesis is altered by ozone in wood of hybrid poplar (Populus tremula×alba). Journal of Experimental Botany, 62(10): 3575-3586. DOI: 10.1093/jxb/err047. |
| Richet N, Tozo K, Afif D, et al., 2012. The response to daylight or continuous ozone of phenylpropanoid and lignin biosynthesis pathways in poplar differs between leaves and wood. Planta, 236(2): 727-737. DOI: 10.1007/s00425-012-1644-8. |
| Stevens GC, Fox JF, 1991. The causes of treeline. Annual Review of Ecology and Systematics, 22: 177-191. DOI: 10.1146/ annurev.es.22.110191.001141. |
| Susiluoto S, Hilasvuori E, Berninger F, 2010. Testing the growth limitation hypothesis for subarctic Scots pine. Journal of Ecology, 98(5): 1186-1195. DOI: 10.1111/j.1365-2745. 2010.01684.x. |
| Turgeon R, 2010. The role of phloem loading reconsidered. Plant Physiology, 152(4): 1817-1823. DOI: 10.1104/pp.110.153023. |
| Vitasse Y, Delzon S, Bresson CC, et al., 2009. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Canadian Journal of Forest Research, 39(3): 1259-1269. |
| Vosa J, Puttena van der PEL, Birchb CJ, 2005. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crops Research, 93(1): 64-73. |
| Wardle P, 1993. Causes of alpine timberline: a review of the hypotheses. In: Alden J, Mastrantonio JL, Odum S (eds.). Forest Development in Cold Climates. New York, NY, USA: Plenum Press, pp. 89-103. |
| Wright IJ, Reich PB, Westoby M, 2001. Strategy shifts in leaf physiology, structure and nutrient content concentration between species of high- and low-rainfall and high- and low-nutrient habitats. Functional Ecology, 15: 423-434. DOI: 10.1046/j.0269-8463.2001.00542.x. |
| Xu ZL, Zhao CY, Feng ZD, 2009. A study of the impact of climate change on the potential distribution of Qinghai spruce (Picea crassifolia) in Qilian Mountains. Acta Ecologica Sinica, 29: 278-285. DOI: 10.1016/j.chnaes.2009.09.004. |
| Xu ZZ, Zhou GS, 2007. Relationship between carbon and nitrogen and environmental regulation in plants under global change-from molecular to ecosystem. Journal of Plant Ecology, 31(4): 738-747. (in Chinese) |
| Yoshida S, Forno D, Cock J, et al., 1976. Determination of sugar and starch in plant tissue. In: Yoshida S (ed.). Laboratory Manual for Physiological Studies of Rice. Philippines: The International Rice Research Institute, pp. 46-49. |
| Zhang P, Wang G, Zhang T, et al., 2010. Responses of foliar δ13C in Sabina przewalskii and Picea crassifolia to altitude and its mechanism in the Qilian Mountains, China. Chinese Journal of Plant Ecology, 34(2): 125-133. DOI: 10.3773/j.issn.1005-264x.2010.02.003. (in Chinese) |
| Zhang T, An LZ, Chen T, et al., 2009. Antioxidative system in leaves of picea crassifolia and sabina przewalskii along an altitudinal gradient. Chinese Journal of Plant Ecology, 33(4): 802-811. DOI: 10.3773/j.issn.1005-264x.2009.04.019. (in Chinese) |
| Zhao R, Dielen V, Kinet JM, et al., 2000. Cosuppression of a plasma membrane H+-ATPase isoform impairs sucrose translocation, stomatal opening, plant growth, and male fertility. Plant Cell, 12(4): 535-546. |
| Zhu WZ, Cao M, Wang SG, et al., 2012. Seasonal dynamics of mobile carbon supply in quercus aquifolioides at the upper elevational limit. PLOS ONE, 7(3): e34213. DOI: 10.1371/journal.pone.0034213. |
 2015, 7
2015, 7


