Article Information
- Shuang Li, HongLang Xiao, YiBen Cheng, Fang Wang. 2015.
- Water use measurement by non-irrigated Tamarix ramosissima in arid regions of Northwest China
- Sciences in Cold and Arid Regions, 7(2): 146-156
- http://dx.doi.org/10.3724/SP.J.1226.2015.00146
Article History
- Received: March 5, 2014
- Accepted: August 20, 2014
2. Key Laboratory of Ecohydrology of Inland River Basin, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
A serious shortage of water resources and a fragile environment characterize the transition zone between the Loess Plateau and the Tengger Desert in northwestern China. In response to national policy, many farml and s have been converted to forest to protect the fragile environment since 1990. Tamarix ramosissima Ledeb. is one of important cultivated species in Northwest China; this shrub exhibits a strong tolerance to sunny conditions, wind erosion, drought and salinity(Qu et al., 2007). This species is mainly distributed in arid-desert areas of Northwest China and has been widely used in s and fixation projects as well as for soil and water conservation. A few studies have been conducted to analyze the drought tolerance(Cleverly et al., 1997), hydraulic traits(Xu and Li, 2006; Thomas et al., 2008; Xu et al., 2011), transpiration and water consumption of Tamarix spp.(Sala et al., 1996; Devitt et al., 1997; Zeng et al., 2002; Glenn and Nagler, 2005; Qu et al., 2007; Xu et al., 2008). These studies analyzing the water consumption characteristics of Tamarix spp. were mainly conducted under good soil water conditions; however, little research has been conducted related to transpiration and the response of non-irrigated T. ramosissima to specific weather factors in an arid region and knowledge related to its bidirectional sap flow at night is almost non-existent.
Heat balance sap flow gauges were used in this study to monitor the stem sap flow under non-irrigated conditions. This technology was improved by Sakuratani(1984) , Baker and Van Bavel(1987) and Steinberg et al.(1989). Currently, sap flow gauges have been commonly used to monitor real-time transpiration in shrubs(Heilman and Ham, 1990; Moore et al., 2008; Yue et al., 2008; Prieto et al., 2010; She et al., 2013). The accuracy of the gauges have been discussed by Senock and Ham(1993) , 1995), Gutierrez et al.(1994) , Grime et al.(1995) , and Angadi et al.(2003). These experiments were mainly conducted in the laboratory, not under field conditions. Although the accuracy of the heat balance method has been compared with other methods(e.g., lysimeter, water balance equation)in the field(Coelho Filho et al., 2005; She et al., 2013), the information on the accuracy of sap flow gauges for T. ramosissima is lacking.
The heat balance sap flow gauges allow transpiration from branches of individual trees to be measured continuously, and it is necessary to develop a suitable scaling procedure for scaling up from sap flow measurements taken from individual stems to plant-level transpiration(Allen and Grime, 1995; Yue et al., 2009). Transpiration of shrubs has been calculated by scaling up from sap flow measurements in a sample of stems to the entire plot using the relationship of sap flow to diameter at breast height, stem cross-section area(Cao et al., 2013), and leaf area(Allen and Grime, 1995; Yue et al., 2008). A few studies have reported that transpiration at st and level can be calculated by the relationship of sap flow with sapwood area, but the correlation is usually used to calculate transpiration of a woody st and (Vertessy et al., 1995; Zhang et al., 2011), and rarely used in the st and -level transpiration of shrub plantations.
This study chose T. ramosissima as the research object, and this species is representative dominant shrub in arid-desert areas of Northwest China. The first objective of this research was to determine the accuracy of stem heat balance sap flow gauges for measuring transpiration in T. ramosissima. The second objective was to analyze the relationship and functional patterns of stem sap flow with meteorological factors during the growing season. The goal was to determine the dominant factors affecting sap flow and provide a preliminarily underst and ing of bidirectional sap flow at night. The third objective was to explore an appropriate scaling procedure for scaling up the sap flow from a sample of stems to an individual plant and to shrub forest. 2 Materials and methods 2.1 Experimental site and plant material
The research area is located in the transition zone between the Loess Plateau and the Tengger Desert in northwestern China, specifically in Sitan Village of Jingtai County, Gansu Province(37°14¢N, 103°48¢E; 1, 828 m a.s.l.). The temperate continent-arid climate of the site has a mean annual temperature of 8.6 °C with extreme low and high temperatures of about −27.3 °C in January and 38.6 °C in July. The annual active accumulated temperature of ≥0 °C is about 3, 614.8 °C, the frost-free period is about 120 d. Mean annual precipitation is about 180 mm, with uneven intra-annual distribution and low rainfall intensity; about 60% of the total rainfall falls from July to September, and winter and spring are very dry. The potential evaporation is 3, 038 mm/a. The wind speed averages 2.0-3.1 m/s, with an instantaneous maximum wind speed of 21.7 m/s; blowing s and weather occurs frequently. Soil types are mainly sierozem and gray-brown desert soil. Since 1990 the local government has converted farml and to forest to protect the fragile environment.
In 2003, the study site was converted from farml and to forest consisting of the shrub T. ramosissima with herbaceous plants such as Suaeda glauca(Bunge)Bunge and Chenopodium album L.. The plants of T. ramosissima were planted in a uniform pattern with 2 m spacing and 4 m between the rows. Mean tree height ofT. ramosissima was 170 cm; mean basal diameter was 3.2 cm. No ground water is available at the site. Water sources for plants mainly include precipitation and residual soil water, without irrigation. Sap flow in T. ramosissima plants, soil water content and meteorological factors were measured from June to September 2013, but the soil water content was not measured in August. Transpiration measured by the rapid weighing method was conducted in September 2013. 2.2 Sap flow measurements
The experiments were conducted in an area dominated by T. ramosissima from June to September 2013. A 100m×100m(1 ha)representative sample site was selected and all vegetation parameters and sap flow measurements were conducted inside this plot. Four to five representative vigorous and healthy T. ramosissima plants were selected. These selected plants were classified as small, medium and large in size to reflect the overall growth of the T. ramosissima plants. We used stem flow gauges(Flow 32, Dynamax Inc., Houston, TX, USA)with the energy balance principle to measure sap flow in the stems of T. ramosissima. Stem diameter was measured withvernier calipers. Each gauge was carefully selected based on the measurement scale of the gauges and the characteristics of the shrubs. To replicate the measurements, two or three sensors were used for each size, and a total of nine to twelve stems were fitted and measured with sap flow gauges.
Gauges were carefully installed following the manufacturer’s instructions. Each gauge was wrapped with multiple layers of aluminum bubble foil insulation to shield it from rain and direct sunlight and reduce the extraneous thermal gradients across the heated section of the stem to a negligible level. Also, shelters were made and sealed with neutral silicate glass cement just above the gauges to prevent water flowing down the stems into the gauges. The outputs from the gauges were monitored every 10 s with programmable data loggers(CR 1000, Campbell Scientific, Logan, UT, USA). The measured signals were averaged every 1 min and recorded the flow rate at 6 min intervals. The gauges were checked and then moved to different stems every 7 to 14 days to avoid plant damage as a result of sensor heating(Kjelgaard et al., 1997). 2.3 Soil water content and the meteorological measurements
Soil samples were taken to a depth of 200 cm at 20 cm intervals in the experimental plot, using a soil auger(5 cm in diameter)every month. Soil water content was calculated by the oven-dry method.
In 2012, an automatic weather station(AWS; Type WS01, Delta-T, Cambridge, UK)was set up about 200 m from the experimental field. Meteorological data were recorded by a data logger at half an hour intervals from June to September. The meteorological factors included wind speed(m/s), rainfall(mm), relative humidity(RH, %), air temperature(Ta, °C), soil temperature(°C), atmospheric pressure(hPa), net solar radiation(kW/m2), photosynthetically active radiation(PAR, mmol), and wind direction. Vapor pressure deficit(VPD, hPa)was calculated based on RH and Ta measurements(Allen et al., 1998). 2.4 Transpiration measurements
A daily transpiration rate experiment was established to test the accuracy of the stem heat balance method for evaluating the transpiration rates of T. ramosissima in the field. The rapid weighing experiment was conducted from 11:00 a.m. to 8:30 p.m. on September 13, 2013 and from 7:00 a.m. to 11:00 a.m. the next day.
Several twigs with leaves were cut from the middle part of the plant canopy, which was adjacent to a plant with attached gauges; these detached twigs were rapidly weighed by electronic balance(0.001 g resolution)within a wind-resistant shelter, recorded as W1(g). After detached twigs were exposed for 2-3 min, the final weight(W2, g)was measured again. Every measurement was completed within 3 min to record the weight loss caused by transpiration. Six repeated measures were used in this experiment. The transpiration rate of T. ramosissima was measured every 20 min by rapid weighing. Because leaves of T. ramosissima are short and bulbous scaly, calculating the leaf area of plants is inconvenient and difficult. Therefore, leaves were removed from the detached twigs after the second weight was measured and the weight of the twigs without leaves was recorded as W3(g). The difference of W1−W3 is the fresh weight of leaves. In addition, leaves were put into envelopes and taken to the laboratory to obtain the net dry weight, Wd(g). The loss of water was expressed using Equation (1):
Tr =(W1−W2)∙60/[(W1−W3)∙t](1)
where Tr is the transpiration rate of detached leaves(g/h), the meaning of each weight parameter in equation is as mentioned above and t is time interval between measurement of W1 and W2(min).
All the leaves were completely removed from the stem with attached gauge on the morning of September 16, 2013, and placed into envelopes. The process was completed by 2-3 people to reduce water loss of leaves. The total fresh weight of leaves and each envelope was recorded as WT(g). Envelopes were oven dried and weighed in the laboratory before the field experiment, with envelope weight recorded as W0(g). The fresh weight of leaves was equal to WT−W0. Stem transpiration rate with attached gauge was calculated by Equation (2):
E = Tr ∙(WT−W0)(2)
where E is the transpiration rate of stems(g/h); Tr as described above; the meaning of each weight parameter in equation is as mentioned above.
Gauge measurements of sap flow rates were compared with rapid weighing measurements of water loss obtained from an electronic balance. The accuracy of the two types of gauges was evaluated in the field test. Gauge error was calculated by Equation (3):
Percent error =(Gauge−Balance)/Balance×100% (3)
where Gauge is sap flow rate(g/h)measured by the gauge, and Balance is transpiration rate(g/h)measured by recursive method and rapid weighing method. 2.5 Data analyses
Partial correlation analyses and stepwise multiple regression analyses were conducted to describe the relationships between diurnal changes in sap flow and significant environmental factors in growing season. All statistical analyses were performed with the SPSS software package(Version 17.0 for Windows, SPSS Inc., USA), with α=0.05 as the threshold for statistical significance. For all the regression analyses, mean observations from at least three sap flow gauges were used. 3 Results 3.1 Measured transpiration and sap flow
Figure 1 illustrates the functional relationships between diurnal sap flow rates measured by two different types of gauges and corresponding transpiration rates measured by the rapid weighing method on September 13 and 14, 2013. For SGA9 and SGB16, the two commonly used gauges throughout the measuring period, a significant linear relationship between sap flow and transpiration rate was observed and correlation coefficients were above 0.90(R2=0.936 for SGA9 in Figure 1a and R2=0.923 for SGB16 in Figure 1b;P <0.001). The slopes of two sets of the linear fits were close to 1(P <0.001). Although the intercept of linear equation was higher for gauge SGB16, it was not significantly different from zero; in particular, the intercept was only 0.631 for gauge SGA9(Figure 1a).
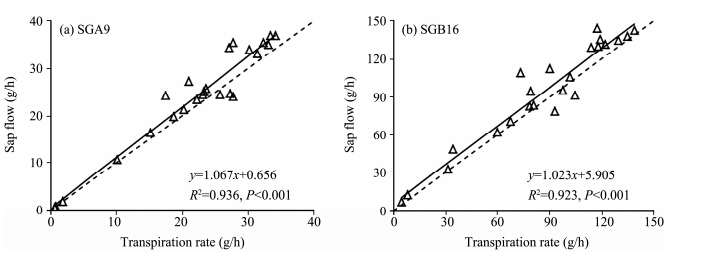 |
| Figure 1 Comparison between heat balance measurements of sap flow in T. ramosissima and rapid weighing measurements of transpiration rate during the daytime in September 2013.(a) and (b)correspond to two different types of gauges(SGA9 and SGB16). The dotted line represents a 1:1 relationship |
One common characteristic of the two gauge types is that sap flow measured by them overestimated water loss over a short period. The percent error was calculated according to Equation (3). The average error was 11.55% for SGA9 and 13.28% for SGB16 over 12 hours. 3.2 Variation of sap flow rate in T. ramosissima under different weather conditions
Figure 2 shows the daily variations of sap flow of stem and branch in T. ramosissima on sunny, cloudy and rainy(drizzle)days from July 23 to 25, 2013. The daily courses of sap flow in both stem and branch were strongly influenced by solar radiation; coincident peaks and troughs were clearly observed under any weather conditions(Figures 2a and 2b). No significant time lag between sap flow and solar radiation was observed. Sap flow still existed in stem and branch at night. The diurnal sap flow pattern was not fully synchronous with the diurnal patterns of relative humidity and VPD on sunny day, but the correlations between them were better on the cloudy and rainy days(Figure 2).
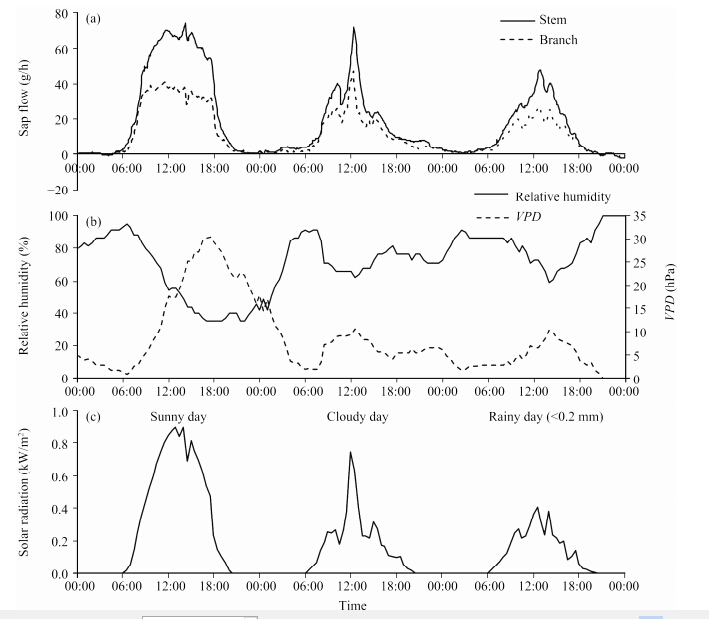 |
| Figure 2 (a)Half-hourly pattern of solar radiation during July 23-25, 2013;(b)Half-hourly patterns of sap flow of stems and branches in T. ramosissima during the same time period. The basal diameter of stem and branch were 22.01 and 15.56 mm, respectively;(c)Half-hourly patterns of relative humidity and vapor pressure deficit(VPD) |
A small amount of negative sap flow occurred from 3:00 a.m. to 5:00 a.m. in the early morning on July 23, and reverse sap flow also occurred on the third day; at this time the relative humidity exceeded 85% with a low VPD(Figures 2a and 2c). 3.3 Relationship between sap flow of T. ramosissima and meteorological factors
Documenting the relationships between sap flow and weather factors would not only demonstrate the effect of weather factors on sap flow, but also be useful to estimate the amount of plant transpiration.
Table 1 summarizes the partial correlation results of mean sap flow rate of T. ramosissima with meteorological factors such as Rns, Ta, RH, VPD and Vw on the sunny day during the measuring period. Rns was always a dominant factor that influenced the sap flow rate of T. ramosissima from June to September(P <0.001). In July, with the exception of Vw, the partially adjusted determinant coefficients of other environmental factors with sap flow rate reached a highly significant level. This occurred mainly because soil water content in the root zone obviously increased as a result of recharge of relatively rich precipitation in this month(Figure 3). Also, plants were in a period of vigorous growth in July, so transpiration was very sensitive to meteorological factors. In August, high winds(>5 m/s)occurred frequently during the daytime; sap flow was negatively correlated with wind speed(P <0.05). Overall, the influence of meteorological factors on stem sap flow can be ranked as: Rns > VPD > Ta > RH > Vw&l t;/ i> during the growing season.
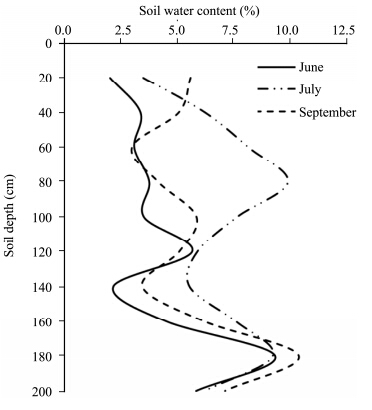 |
| Figure 3 Soil water content from 0 to 200 cm |
| Meteorological factors | Partial correlation coefficient | June 2013 | July 2013 | August 2013 | September 2013 |
| Net solar radiation, Rns | R2 | 0.828*** | 0.941*** | 0.790*** | 0.900*** |
| Air temperature, Ta | R2 | 0.412** | 0.808*** | 0.187 | 0.423 |
| Relative humidity, RH | R2 | −0.409** | −0.782*** | 0.300 | −0.383 |
| Vapor pressure deficit, VPD | R2 | 0.397** | 0.488** | 0.413** | 0.544*** |
| Wind speed, Vw | R2 | 0.166 | −0.065 | −0.403** | 0.176 |
| Note: means significance(two-tailed)of P <0.10; **, P <0.05; ***, P <0.01. | |||||
The multiple linear regression of sap flow rate of T. ramosissima with Rns, Ta, RH, VPD and Vw was programmed by SPSS using the stepwise regression method to help researchers comprehend the combined influence of weather factors on sap flow(Table 2). The results show that stem sap flow rate in T. ramosissima was only the result of Rns in June, and it was the result of Rns and Ta in July with optimal fitting result among these months. The fitting result, using Rns and VPD in August, was relatively poor among these months, and the sap flow rate was the result of Rns, Ta and VPD in September. The sap flow rate mainly depended on the Rns in any month. Compared with measured sap flow, the error of the predicted value from June to September was 6.51%, 0.72%, 14.00%, −0.78% over 12 hours, respectively.
| Month | Regression equation | Correlation coefficient(R2) | F value | Significance |
| June | SF=28.197+45.842Rns | 0.774 | 92.615 | 0.000 |
| July | SF=−65.314+26.515Rns+3.561Ta | 0.975 | 516.910 | 0.000 |
| August | SF=16.705+24.145Rns−0.586VPD | 0.743 | 36.221 | 0.000 |
| September | SF=−38.729+70.712Rns+3.978Ta−1.737VPD | 0.933 | 97.535 | 0.000 |
| Note: Rns is the net solar radiation, Ta is air temperature, VPD is vapor pressure deficit. | ||||
Figure 4 shows the hourly variation and magnitude of sap flow expressed in different units in same three stems on September 13, 2013. Trends of sap flow in the three stems resembled each other with clearly coincident peaks and troughs, no matter what units were used. Compared with patterns of sap flow in three T. ramosissima stems expressed by grams per hour and by stem cross-sectional area(Figures 4a and 4b), the three time series exactly overlaid one another when the sap flows were normalized by their leaf dry weights(Figure 4c), which showed that sap flow per unit leaf dry weight was much less variable than sap flow per stem cross-sectional area. Sap flow rates were evidently linked to leaf dry weight and greater sap flows corresponded to larger leaf dry weight. A more detailed analysis showed that a strong positive linear relationship existed between diurnal sap flow and leaf dry weight(R2=0.965, P <0.001), significant differences appeared within a diameter class and cross-sectional area class(Figure 5). For example, sap flow of the stem with a basal diameter 12.09 mm was larger than that of stem with a basal diameter 12.16 mm(Figure 4a), indicating that there was no relationship between stem diameter and sap flow. Similar results can be obtained between stem cross-section and sap flow. This suggested that leaf dry weight was more reliable than stem diameter and cross-section area for the extrapolation.
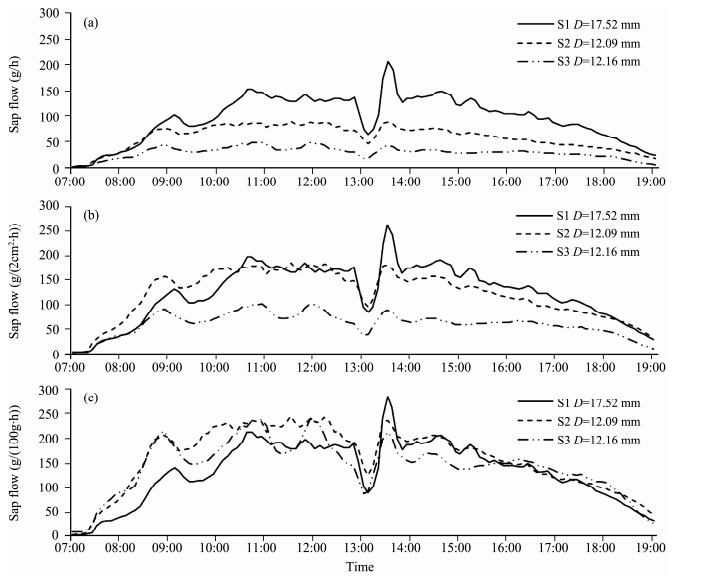 |
| Figure 4 The hourly variation of sap flow expressed using different units in three T. ramosissima stems on September 13, 2013.(a)Sap flow(g/h)in three stems(S1, S2, and S3)with different diameters(D = 17.52, 12.09 and 12.16 mm), respectively;(b) and (c)sap flow normalized by stem cross-sectional area(g/(cm2∙ h)) and leaf dry weight(g/(g∙h))in the same stems over the same period of time, respectively. The leaf dry weight of stem S1, S2 and S3 were 72.23, 37.07 and 20.01 g, respectively |
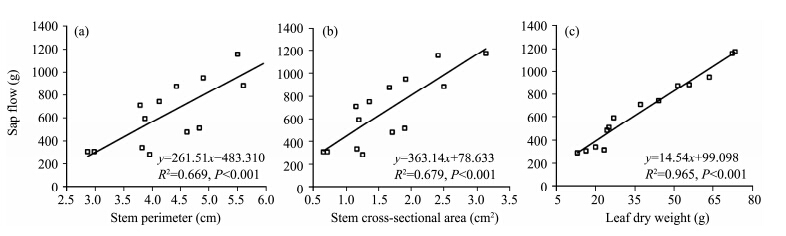 |
| Figure 5 Relationship between accumulated sap flows during 07:00 to 19:00 and stem perimeter, cross-sectional area and leaf dry weight on September 13, 2013 |
Our results showing an overestimation of transpiration by the sap flow method, compared with the rapid weighing method(Figure 1), are similar to those of authors who compared sap flow with transpiration measured by gravimetric measurement in a laboratory(Ham, 1990; Angadi et al.(2003)). Our results contradict those of authors who concluded an underestimation of transpiration by the sap flow method, compared with the weighing lysimeter(Shackel et al., 1992; Yue et al., 2008). The overestimation of water loss by sap flow is mainly attributed to external conditions. In arid regions, vegetation is typically sparse. Sap flow gauges are sensitive to external conditions(Gutierrez et al., 1994; Grime and Sinclair, 1999), their accuracy would be disturbed by solar radiation in the areas with low vegetation coverage(Gutierrez et al., 1994; Senock and Ham, 1995), but if sufficient insulation and shielding are done, the effect of large fluctuations in ambient temperature on gauges can be effectively minimized(Shackel et al., 1992; Gutierrez et al., 1994). The discrepancy between sap flow method and weighing lysimeter method is mainly due to that the contribution of evaporation from the soil surface was taken into account by the weighed lysimeter, but not by the sap flow method(Allen, 1990).
In most literature, an error of <10% was reported in averages through 15 min to 24 hours for herbaceous and woody plants(Weibel and DeVos, 1994). The average error was 11.55% for SGA9 and 13.28% for SGB16 over 12 hours in this paper. Angadi et al.(2003) found the mean error of sap flow was 11.9% over 1 hour for the stem with diameter of 9 mm and was 11.3% over an entire day, which is similar to our results. 4.2 The hourly variation of sap flow
Partial correlation analysis between sap flow and meteorological factors indicated that solar radiation was always the dominant element affecting the variation of sap flow in T. ramosissima during the period from June to September. For example, the trough in sap flow rate at around 1:00 a.m. corresponded to the low value of solar radiation(Figures 4 and 6). The influence of meteorological factors on stem sap flow could be ranked as: Rns > VPD > Ta > RH > Vw. Qu et al.(2007)produced a similar conclusion and ranked the main meteorological factors affecting the sap flux of T. elongate Ledeb. trees as Rns > Ta > VPD > RH > Vw. Moore et al.(2008)analyzed the sensitivity of sap flux in T. chinensis Loureiro to VPD and found a positive relationship between sap flow and VPD during the daytime(R2=0.54, P <0.001)in September and October, which is very close to our results showing that the partial correlation coefficient between sap flow and VPD was 0.544(P=0.007)in September. A negative correlation between sap flow and wind speed in August might result from stomatal closure induced by high wind(Wang et al., 2006).
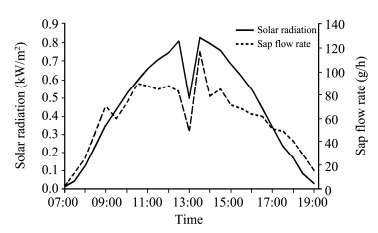 |
| Figure 6 The hourly variations of mean sap flow and solar radiation on September 13, 2013. The mean sap flow here refers to the mean value of sap flows in three stems(S1, S2, and S3) |
The accumulated precipitation in June, July and August was 30.0, 50.4 and 14.8 mm, respectively. On the basis of monthly rainfall, soil water content(Figure 3), and the relationships between sap flow and meteorological factors(Tables 1 and 2), it can be concluded that when plant suffers from water stress, sap flow would have reduced sensitivity to meteorological factors, and the corresponding pattern would also change accordingly. Soil water content would be one of the main factors affecting plant sap flow in a very dry season. No significant time lag was observed between sap flow and solar radiation in the drought resistant shrub T. ramosissima; this is consistent with the results of Yue et al.(2008) , although a time lag did exist in other drought resistant shrubs(Zhao and Liu, 2010; She et al., 2013).
Table 3 presents accumulated sap flow of stem and branch under different weather conditions from July 18 to 25, 2013. Accumulated sap flow was normalized by stem cross-sectional area(g/cm2). The stem and the branch here were the same as those mentioned in Figure 2. As shown in Table 3, sap flows of both stem and branch in daytime could be ranked as sunny day > cloudy day > rainy day. The proportion of positive flow at night, accounting for diurnal sap flow, ranged from 4.17% to 14.14% in stem and from 6.48% to 17.17% in branch. Bucci et al.(2004)reported that sap flow at night during the dry season could contribute 13% to 28% of total daily transpiration. Other studies have demonstrated that nighttime transpiration of woody plants can exceed 20% of daily transpiration(Fisher et al., 2007; Moore et al., 2008). Nighttime sap flow was extremely important for plant survival; nighttime and predawn sap flow values could provide an indication of a plant’s water deficit that accumulated during the previous day(Nadezhdina, 1999).
| Weather | Date | Sap flow of stem(g/cm2) | Sap flow of branch(g/cm2) | |||||
| Daytime | Nighttime | Daytime | Nighttime | |||||
| Positive | Negative | Positive | Negative | |||||
| Cloudy | 18 | 89.035 | 4.676 | 0.000 | 100.025 | 4.683 | 0.000 | |
| Sunny | 19 | 169.283 | 11.331 | 0.000 | 172.734 | 10.192 | 0.000 | |
| Sunny | 20 | 158.405 | 7.063 | 0.000 | 164.690 | 6.633 | 0.000 | |
| Sunny | 21 | 155.352 | 8.305 | 0.000 | 182.566 | 7.770 | 0.000 | |
| Sunny | 22 | 163.657 | 8.474 | 0.000 | 183.020 | 5.102 | 0.000 | |
| Sunny | 23 | 175.344 | 2.470 | 0.111 | 201.334 | 3.903 | 0.124 | |
| Cloudy | 24 | 88.736 | 10.028 | 0.000 | 120.924 | 10.924 | 0.000 | |
| Rainy | 25 | 76.233 | 3.255 | 0.627 | 96.967 | 6.124 | 0.523 | |
| Rainy | 26 | 30.333 | 2.146 | 2.755 | 26.489 | 3.512 | 1.869 | |
| Rainy | 27 | 75.457 | 4.401 | 0.302 | 83.594 | 6.666 | 0.438 | |
| Cloudy | 28 | 124.358 | 5.903 | 0.000 | 142.782 | 7.384 | 0.000 | |
| Note: The daytime is from 06:30 to 20:30, and the rest of the day is nighttime. | ||||||||
Table 3 indicates that obvious reverse sap flow occurred on rainy days. The magnitude of reverse sap flow for the stem accounted for 4.62% of diurnal sap flow, and 5.44% of diurnal sap flow for the branch. When negative sap flow occurred, the common meteorological feature was that high air humidity occurred, and the phenomenon has been mentioned in Figure 2. Phenomena of foliar water uptake and reverse sap flow in stems under high atmospheric humidity conditions have been reported by Burgess and Dawson(2004) , Hultine et al.(2004) , Fisher et al.(2007) and Berry and Smith(2014). High relative humidity is a necessary but not required condition for the occurrence of negative sap flow. The formation of negative sap flow was also influenced by the hydraulic potential gradient between soil, plant(root, stem and leaf) and atmosphere. Water can move into and through plants in both positive and negative directions when a proper gradient is established(Jensen et al., 1961). 4.3 Correlation between sap flow and sapwood area of stem
Figure 5 shows that linear correlation coefficients between daily sap flow and stem cross-sectional perimeter and area were 0.669 and 0.679, respectively, below the correlation coefficient(0.739)between daily sap flow and stem sapwood area(Figure 7a). It is neither possible nor desirable to measure the sapwood area of each stem in the entire sample plot directly. Therefore, the relationship between stem diameter and sapwood area was established, and it was a very significant power function relationship(Figure 7b). St and transpiration could be obtained on the basis of the relationship between sap flow and sapwood area; this method has been widely used to estimate forest transpiration, but has been rarely used to estimate shrub transpiration. Based on the methods of Zhang et al.(2011) and Cao et al.(2013) , transpiration rates of several T. ramosissima plants were calculated by taking sapwood area and cross-sectional area as extended parameters, respectively. Compared with the transpiration measured by rapid weighing method, the average error was 8.19% for transpiration calculated by taking sapwood area, and 10.78% for transpiration calculated by taking cross-sectional area. So, using sapwood area as an extended parameter to estimate shrub transpiration provides more precise results. We believe the diurnal transpiration rate in T. ramosissima can be forecasted through the multiple linear correlation equation between sap flow and meteorological factors, and transpiration of an individual plant or shrub may be estimated by taking sapwood area as an extended parameter.
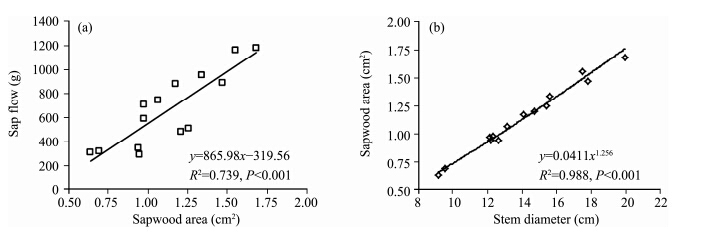 |
| Figure 7 Relationship of sapwood area with sap flow, stem diameter and leaf dry weight on September 13, 2013 |
The heat balance sap flow gauges were evaluated for measuring transpiration of T. ramosissima. Sap flows measured by gauge SGA9 and SGB16 had a significant linear relationship with corresponding transpiration measured by the rapid weighing method. Sap flow of T. ramosissima was closely related to above ground biomass, meteorological factors and soil water content. The linear correlation coefficient between diurnal sap flow and leaf dry weight reached 0.965(P <0.001). Solar radiation was always a dominant factor that influenced the sap flow rate of T. ramosissima from June to September(P <0.001), but soil water content would be one of the main factors affecting plant sap flow in a very dry season. The daily patterns of sap flow in T. ramosissima varied under different weather conditions. Bidirectional sap flows were monitored at night. The proportion of positive flow at night, accounting for diurnal sap flow, ranged from 4.17% to 14.14% in stem, and from 6.48% to 17.17% in branch. Negative sap flow at night corresponded to high relative humidity(over 85%), but high relative humidity is necessary but not always sufficient condition for negative sap flow to occur.
Compared with measured sap flow, the average error of predicted value over 12 hours, obtained from multiple linear regression equations between sap flow and meteorological factors, was 6.512%, 0.723%, 14.003%, −0.783% from June to September, respectively. According to the functional equations between sap flow and meteorological factors as well as sapwood area, transpiration of an individual plant, and even the st and -level transpiration, can be calculated through extrapolation.
Acknowledgment:This study is financially supported by the National Natural Science Foundation of China(No. 91125025).
| Allen RG, Pereira LS, Raes D, et al., 1998. Crop evapotranspira-tion–Guidelines for computing crop water requirements–FAO Irrigation and drainage paper 56. FAO, Rome, 300: 6541. |
| Allen SJ, 1990. Measurement and estimation of evaporation from soil under sparse barley crops in northern Syria. Agricultural and Forest Meteorology, 49(4): 291–309. DOI: 10.1016/0168-1923(90)90003-O. |
| Allen SJ, Grime VL, 1995. Measurements of transpiration from savannah shrubs using sap flow gauges. Agricultural and Forest Meteorology, 75(1): 23–41. DOI: 10.1016/0168-1923 (94)02201-T. |
| Angadi SV, Cutforth HW, McConkey BG, 2003. Determination of the water use and water use response of canola to solar radiation and temperature by using heat balance stem flow gauges. Ca-nadian Journal of Plant Science, 83(1): 31–38. DOI: 10.4141/P02-022. |
| Baker JM, van Bavel CHM, 1987. Measurement of mass flow of water in the stems of herbaceous plants. Plant Cell and Envi-ronment, 10(9): 777–782. DOI: 10.1111/1365-3040.ep11604765. |
| Berry ZC, Smith WK, 2014. Experimental cloud immersion and foliar water uptake in saplings of Abies fraseri and Picea rubens. Trees, 28(1): 115–123. DOI: 10.1007/s00468-013-0934-5. |
| Bucci SJ, Scholz FG, Goldstein G, et al., 2004. Processes preventing nocturnal equilibration between leaf and soil water potential in tropical savanna woody species. Tree Physiology, 24(10): 1119–1127. DOI: 10.1093/treephys/24.10.1119. |
| Burgess SSO, Dawson TE, 2004. The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration. Plant Cell and Environment, 27(8): 1023–1034. DOI: 10.1111/j.1365-3040.2004.01207.x. |
| Cao XM, Chen X, Wang JL, et al., 2013. Water consumption and transpiration of non-irrigated Haloxylon ammodendron in hin-terland of Taklimakan desert. Arid Land Geography, 36(2): 292–302. DOI: 1000-6060(2013)02-0292-11. |
| Cleverly JR, Smith SD, Sala A, et al., 1997. Invasive capacity of Tamarix ramosissima in a Mojave Desert floodplain: the role of drought. Oecologia, 111(1): 12–18. DOI: 10.1007/s004420050202. |
| Coelho Filho MA, Angelocci LR, Campeche LFSM, et al., 2005. Field determination of young acid lime plants transpiration by the stem heat balance method. Scientia Agricola, 62(3): 240–247. DOI: 10.1590/S0103-90162005000300007. |
| Devitt DA, Salal A, Mace KA, et al., 1997. The effect of applied water on the water use of saltcedar in a desert riparian envi-ronment. Journal of Hydrology, 192(1): 233–246. DOI: 10.1016/S0022-1694(96)03105-8. |
| Fisher JB, Baldocchi DD, Misson L, et al., 2007. What the towers don’t see at night: nocturnal sap flow in trees and shrubs at two AmeriFlux sites in California. Tree Physiology, 27(4): 597–610. DOI: 10.1093/treephys/27.4.597. |
| Glenn EP, Nagler PL, 2005. Comparative ecophysiology of Tamarix ramosissima and native trees in western US riparian zones. Journal of Arid Environments, 61(3): 419–446. DOI: 10.1016/j.jaridenv.2004.09.025. |
| Grime VL, Morrison JIL, Simmonds LP, 1995. Including the heat storage term in sap flow measurements with the stem heat balance method. Agricultural and Forest Meteorology, 74(1): 1–25. DOI: 10.1016/0168-1923(94)02187-O. |
| Grime VL, Sinclair FL, 1999. Sources of error in stem heat balance sap flow measurements. Agricultural and Forest Meteorology, 94(2): 103–121. DOI: 10.1016/S0168-1923(99)00011-8. |
| Gutierrez MV, Harrington RA, Meinzer FC, et al., 1994. The effect of environmentally induced stem temperature gradients on transpiration estimates from the heat balance method in two tropical woody species. Tree Physiology, 14(2): 179–190. DOI: 10.1093/treephys/14.2.179. |
| Ham JM, 1990. Dynamics of a heat balance stem flow gauge during high flow. Agronomy Journal, 82(1): 147–152. DOI: 10.2134/agronj1990.00021962008200010032x |
| Heilman JL, Ham JM, 1990. Measurement of mass flow rate of sap in Ligustrum japonicum. Hort Science, 25(4): 465–467. |
| Hultine KR, Scott RL, Cable WL, et al., 2004. Hydraulic redistri-bution by a dominant, warm-desert phreatophyte: Seasonal patterns and response to precipitation pulses. Functional Ecology, 18(4): 530–538. DOI: 10.1111/ j.0269-8463.2004.00867.x. |
| Jensen RD, Taylor SA, Wiebe HH, 1961. Negative transport & resistance to water flow through plants. Plant Physiology, 36(5): 633–638. |
| Kjelgaard JF, Stockle CO, Black RA, et al., 1997. Measuring sap flow with the heat balance approach using constant and variable heat inputs. Agricultural and Forest Meteorology, 85(3): 239–250. DOI: 10.1016/S0168-1923(96)02397-0. |
| Moore GW, Cleverly JR, Owens MK, 2008. Nocturnal transpiration in riparian Tamarix thickets authenticated by sap flux, eddy covariance and leaf gas exchange measurements. Tree Physi-ology, 28(4): 521–528. DOI: 10.1093/treephys/ 28.4.521. |
| Nadezhdina N, 1999. Sap flow index as an indicator of plant water status. Tree Physiology, 19(13): 885–891. DOI: 10.1093/treephys/19.13.885. |
| Prieto I, Kikvidze Z, Pugnaire FI, 2010. Hydraulic lift: soil processes and transpiration in the Mediterranean leguminous shrub Retama sphaerocarpa (L.) Boiss. Plant and Soil, 329(1–2): 447–456. DOI: 10.1007/s11104-009-0170-3. |
| Qu YP, Kang SZ, Li FS, et al., 2007. Xylem sap flows of irrigated Tamarix elongata Ledeb and the influence of environmental factors in the desert region of Northwest China. Hydrological Process, 21(10): 1363–1369. DOI: 10.1002/ hyp.6314. |
| Sakuratani T, 1984. Improvement of the probe for measuring water flow rate in intact plants with the stem heat balance method. Journal of Agricultural Meteorology, 40(3): 273–277. |
| Sala A, Smith SD, Devitt DA, 1996. Water use by Tamarix ramo-sissima and associated phreatophytes in a Mojave Desert floodplain. Ecological Application, 6(3): 888–898. DOI: 10.2307/2269492. |
| Senock RS, Ham JM, 1993. Heat balance sap flow gauge for small diameter stems. Plant Cell and Environment, 16(5): 593–601. DOI: 10.1111/j.1365-3040.1993.tb00908.x. |
| Senock RS, Ham JM, 1995. Measurements of water use by prairie grasses with heat balance sap flow gauges. Journal of Range Management, 48(2): 150–158. DOI: 10.2307/4002803. |
| Shackel KA, Johnson RS, Medawar CK, et al., 1992. Substantial errors in estimates of sap flow using the heat balance technique on woody stems under field conditions. Journal of American Society for Horticltural Science, 117(2): 351–356. |
| She DL, Xia YQ, Shao MA, et al., 2013. Transpiration and canopy conductance of Caragana korshinskii trees in response to soil moisture in sand land of China. Agroforestry Systems, 87(3): 667–678. DOI: 10.1007/s1045 7-012-9587-4. |
| Steinberg S, van Bavel CHM, McFarland MJ, 1989. A gauge to measure mass flow rate of sap in stems and trunks of woody plants. Journal of American Society for Horticultureal Science, 114(3): 466–472. |
| Thomas FM, Foetzki A, Gries D, et al., 2008. Regulation of the water status in three co-occurring phreatophytes at the southern fringe of the Taklamakan Desert. Journal of Plant Ecology, 1(4): 227–235. DOI: 10.1093/jpe/rtn023. |
| Vertessy RA, Benyon RG, O’sullivan SK, et al., 1995. Relationships between stem diameter, sapwood area, leaf area and transpiration in a young mountain ash forest. Tree Physiology, 15(9): 559–567. DOI: 10.1093/treephys/15.9.559. |
| Wang RH, Ma LY, Li LP, et al., 2006. Temporal and spatial varia-tions of stem sap flow of Acer truncatum Bunge. Journal of Beijing Forestry University, 28(2): 12–18. DOI: 1000-1522(2006). |
| Weibel FP, De Vos JA, 1994. Transpiration measurements on apple trees with an improved stem heat balance method. Plant and Soil, 166(2): 203–219. DOI: 10.1007/BF00008334. |
| Xu GQ, Li Y, Xu H, 2011. Seasonal variation in plant hydraulic traits of two co-occurring desert shrubs, Tamarix ramosissima and Haloxylon ammodendron, with different rooting patterns. Ecological Research, 26(6): 1071–1080. DOI: 10.1007/s11284-011-0858-8. |
| Xu H, Li Y, 2006. Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant and Soil, 285(1–2): 5–17. DOI: 10.1007/s11104-005-5108-9. |
| Xu XY, Sun BP, Ding GD, et al., 2008. Sap flow patterns of three main sand-fixing shrubs and their responses to environmental factors in desert areas. Acta Ecologica Sinica, 28(3): 895–905. DOI: 1000-0933(2008)03-895-11. |
| Yue GY, Zhao HL, Zhang TH, et al., 2008. Evaluation of water use of Caragana microphylla with the stem heat-balance method in Horqin Sandy Land, Inner Mongolia, China. Agricultural and Forest Meteorology, 148(11): 1668–1678. DOI: 10.1016/j.agrformet.2008.05.019. |
| Yue GY, Zhao HL, Zhang TH, et al., 2009. Estimation of transpi-ration in communities dominated by shrub Caragana micro-phylla. Chinese Journal of Plant Ecology, 33(3): 508–515. DOI: 10.3773/j.issn.1005-264x.2009.03.010. |
| Zeng FJ, Foetzki A, Li XY, et al., 2002. A preliminary study on the effect of irrigation on water physiology of Tamarix ramosissima in Celeoasis. Chinese Journal of Applied Ecology, 13(7): 849–853. DOI: 1001-9332(2002)07-0849-05. |
| Zhang YT, Liang FC, Chang SL, et al., 2011. Scaling up for trans-piration of Pinaceae schrenkiana stands based on 8 hm2 per-manent plots in Tianshan Mountains. Acta Ecologica Sinica, 31(2): 3330–3339. |
| Zhao W, Liu B, 2010. The response of sap flow in shrubs to rainfall pulses in the desert region of China. Agricultural and Forest Meteorology, 150(9): 1297–1306. DOI: 10.1016/ j.agrformet.2010.05.012. |
 2015, 7
2015, 7


