Article Information
- Emmanuel F. Isola, Olusanya A. Olatunji, Akinjide M. Afolabi, Ademayowa A. Omodara. 2015.
- Heavy metal accumulation in the above-ground vegetation and soil around an iron smelting factory in Ile-Ife, southwestern Nigeria
- Sciences in Cold and Arid Regions, 7(2): 121-127
- http://dx.doi.org/10.3724/SP.J.1226.2015.00121
Article History
- Received: June 22, 2014
- Accepted: October 28, 2014
In recent years, significant attention has been paid to the problems of environmental contamination by a wide variety of chemical pollutants, including heavy metals(El-Demerdash and Elagamy, 1999). These chemicals arise from increasing levels of industrialization and urbanization(Filazi et al.,2003). Environmental pollution causes biochemical and physiological damages which lead to reduction of vegetation growth and yield(Treshow, 1984). It can also have damaging effects on the plants themselves and may become a health hazard to humans and animals(Nayak et al.,2012)when it is above a certain concentration and over a narrow range(Sterritt and Lester, 1980; Babich et al., 1982). Plant injury caused by environmental pollution is most common near large cities and industrial facilities such as smelters, refineries, thermal power plants, highways, incinerators, refuse dumps, and pulp and paper mills(Nayak et al.,2012). Heavy metals(Cu, Cd, Cr, Hg, Pb, and Zn)above threshold levels have strong negative effects on the soil capacity and can inhibit mineralization of soil and synthesis of organic acids essential for plants(Cvetanovska et al.,2005). Heavy metal toxicity has an inhibitory effect on plant growth, enzymatic activity, photosynthetic activity, and accumulation of other nutrient elements(Gune et al.,2004), and also threatens human health through its effects on the hygiene quality of food. Contamination of the environment by heavy metals is viewed as an international problem because of its effects on the ecosystems in most countries. In Nigeria, the situation is no better as the activities of most industries and populace regarding waste disposal and management usually lead to increasing levels of pollution in the environment(Yahaya et al.,2009). Nadal et al.(2004) and Onder et al.(2007) observed that the most economical and reasonable method for monitoring heavy metals in the atmosphere is to assess soil and vegetation samples, which have been widely employed as cumulative matrices of long- and short-term exposure, respectively, to environmental pollutants.
The literature indicates that studies have been conducted on pollution of some areas in southwestern Nigeria, but nothing has been documented to date on the heavy metal levels emanating from the industrial area in Ile-Ife, and their possible accumulation in local soil and vegetation. Therefore, it is important to investigate the level of heavy metals in soil and vegetation around this steel processing facility in order to establish baseline data which can be used for assessing their impact in the area.
2 Materials and methods 2.1 Study site
This study was conducted around an iron smelting factory located at the Fashina Area, Ile-Ife, Osun State. The factory is located at 7°29′43.3″N-7°29′31.6ʺN, 4°28′31.2ʺE-4°28′32.2ʺE. The factory was established in 2010 and is located along the Ife-Ibadan expressway. The site is a typical tropical forest, although modified as a result of human activities. It is a tropical zone with two prominent seasons, the rainy and the dry seasons, with average annual rainfall of 1, 413 mm and mean annual temperature of 22.5-32.4 °C. 2.2 Sampling
Samples of the two most common herbaceous species(Chromolaena odorata and Aspilia africana)around the factory were r and omly collected at 10 m away from the wall of the factory. The plants were selected as significant representative species of the annual vegetation of the area and were collected simultaneously with the soil in which the plants grow. The plant species were oven-dried at 80 °C for 24 h until a constant weight was reached, and were then ground for chemical analyses. Soil samples were r and omly collected at 0-15-cm depths around the vicinity of the factory where the plant species were collected, because most of the root nutrients, including that of large trees, occur in this zone(Stewart et al.,1974). Control samples for the plant species were collected at 2 km away from the factory. In the laboratory, the soil was sieved to obtain a more homogenous distribution and to remove small stones, roots, and large organic residues. 2.3 Methodology
The ground plant samples were digested using the mixed acid(nitric-perchloric-sulphuric)digestion procedure of Allen et al.(1974) . The 0.2 g of each ground sample was digested in 7.0 mL of the digestion mixture. The digested samples, as well as the soil samples, were analyzed for N, P, K, C, Zn, Pb, Cd, Ni, and Cr in order to test for the rate of nutrient accumulation as well as the presence of heavy metals, using an atomic absorption spectrophotometer. The data obtained were subjected to appropriate descriptive and inferential statistical analyses to compare the differences in the seasonal concentration of all the elements in the selected plant species and the soil. 3 Results and discussion
The results of the physiochemical properties of the soil samples in the dry and rainy seasons are summarized in Table 1. In the present study, the soils were characterized as slightly acidic, with pH values of 6.23±0.24 in the dry season and 6.10±0.16 in the rainy season. The soil pH observed in this study for the two seasons fell within the range reported by Oyanuga(1979)for soil within the Ife area, who found that the general surface horizon was neutral to weakly acidic in reaction. It also fell within the recommended pH range for optimal nutrient availability in soil, which is 6.0-7.5(Fagbote and Olanipekun, 2011). The seasonal pH variation observed in these results may also be partially attributed to the change in concentration of the salt in the soil solution due to precipitation(rainfall), which leads to leaching of most basic cations in the soil.
| Parameter | Dry season | Rainy season |
| pH | 6.23 ± 0.24a | 6.10 ± 0.16a |
| Total nitrogen(%) | 2.71 ± 0.22a | 0.22 ± 0.01b |
| Total phosphorus(mg/kg) | 59.42 ± 3.45b | 65.13 ± 2.57a |
| Potassium(cmol/kg) | 0.12 ± 0.00 | 0.22 ± 0.02a |
| Organic matter(%) | 3.61 ± 0.53a | 2.33 ± 0.20b |
| Cadmium(mg/kg) | 0.10 ± 0.00b | 0.32 ± 0.02a |
| Lead(mg/kg) | 0.53 ± 0.16b | 1.10 ± 0.07a |
| Chromium(mg/kg) | 0.02 ± 0.00a | 0.07 ± 0.00b |
| a, b The means within each row with different superscripts are significantly different(P <0.05). | ||
There was a significant difference(P <0.05)in the percentage content of total N, P, K, and organic matter values in the soil samples collected in the two seasons. The percentage content of total N and organic matter were higher during the dry season, whereas the P and K were higher during the rainy season. The higher concentration of N during the dry season followed the same trend as the organic matter because N is usually stored as a major component of soil organic matter from which it is released by mineralization. The relative reduction of N in the rainy season could be ascribed to poor aeration due to soil wetness or lack of pore space, which slows mineralization process(Ilechukwu and Leo, 2013). Also, the higher P and K observed during the rainy season clearly indicated the solubility of these two elements. There was a significant difference(P <0.05)in the Cd, Pb, and Cr values in the soil samples collected in the two seasons, but the observed values were lower than the known toxic thresholds(Eduardo et al.,2010). The Cd, Pb, and Cr contents in soil samples from the rainy season collection were significantly higher(P <0.05)than those in the d ry season. This implies that the majority of these heavy metals were more readily available during the rainy season than the dry season. This was similar to the observation of Kabir and Bouhadjera(2011) in their study of the effects of heavy metal pollution in soil and plants in the industrial area of western Algeria.
The results for the total N, total P, organic C, and K contents of Aspilia africana and Chromolaena odorata are represented in Figures 1-4. The percentage concentrations of N, P, C, and K were higher in Chromolaena odorata than in Aspilia africana in the polluted site and the control area during the rainy season. The uptake and accumulation of mineral elements varied greatly among the plant species and was primarily dependent on the plant species, its inherent, and the soil quality(Chunilall et al.,2005). In the dry season, the C percentage concentration was higher in Aspilia africana, whereas the other elements followed the trend observed in the rainy season. The varying nutrient composition observed in this study depended, among other factors, on their structural compositions, which varied among the species(Hockensmith et al.,1997). The results also showed a progressive decrease in the percentage concentration of the elements tested, with C > P > N in both Aspilia africana and Chromolaena odorata. However, Table 2 shows that there was no significant difference in the concentration of all the analyzed elements in both seasons. This showed that the concentrations of N, P, K, and organic C in both Aspilia africana and Chromolaena odorata were not affected by season.
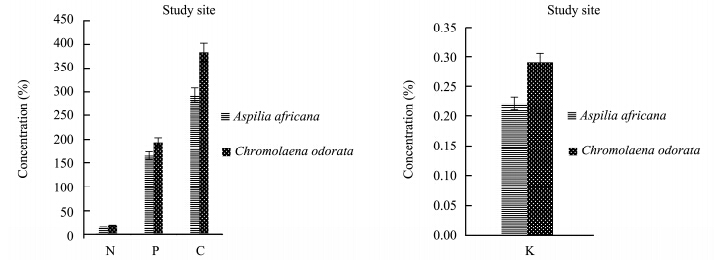 |
| Figure 1 N, P, C, and K concentrations in both Aspilia africana and Chromolaena odorata around the iron smelting factory in the rainy season |
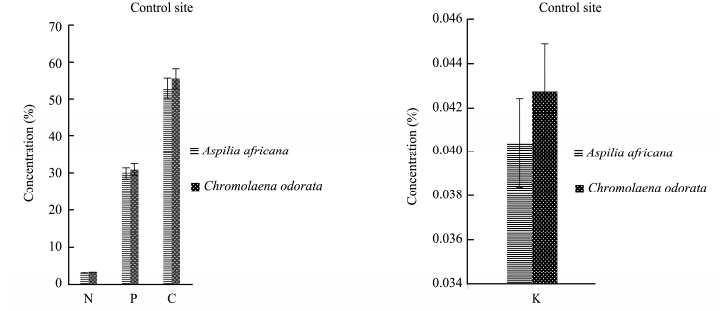 |
| Figure 2 N, P, C, and K concentrations in both Aspilia africana and Chromolaena odorata at the control site in the rainy season |
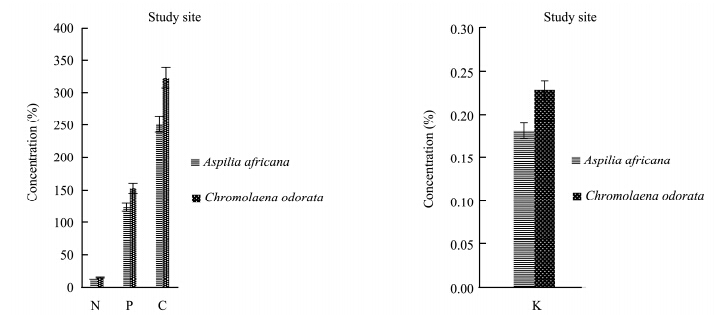 |
| Figure 3 N, P, C, and K concentrations in both Aspilia africana and Chromolaena odorata around the iron smelting factory in the dry season |
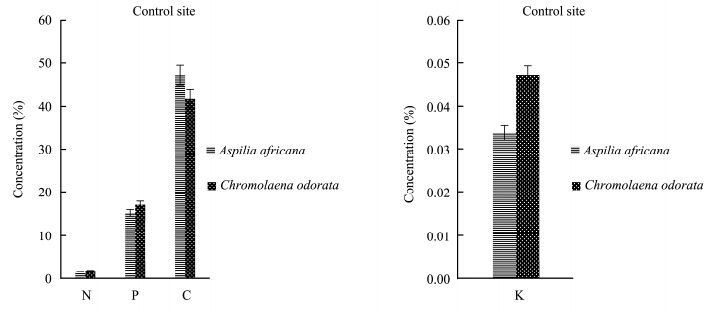 |
| Figure 4 N, P, C, and K concentrations in both Aspilia africana and Chromolaena odorata at the control site in the dry season |
| Plant species | N(g/kg) | P(mg/kg) | K(mg/kg) | C(g/kg) | |||||||
| Dry season | Rainy season | Dry season | Rainy season | Dry season | Rainy season | Dry season | Rainy season | ||||
| Aspilia africana | 2.64±0.44a | 2.95±0.37a | 24.70±4.10a | 27.57±3.50a | 0.03±0.00a | 0.03±0.00a | 50.34±2.98a | 49.02±1.80a | |||
| Chromolaena odorata | 2.73±0.22a | 2.59±0.39a | 25.50±2.06a | 24.23±3.68a | 0.03±0.00a | 0.03±0.00a | 53.96±4.29a | 47.96±6.92a | |||
| a, b The means within each column with different superscripts are significantly different(P <0.05). | |||||||||||
Evaluation of heavy metals in the present study showed that heavy metal concentrations in the plants varied with the plant species and also with the season, and were also lower than national environmental quality st and ard range. The concentration of zinc(Zn)was higher in Aspilia africana in both the polluted site and the control site in the rainy season, while the concentrations of other heavy metals were observed to be higher in Chromolaena odorata(Figures 5-6). This means that Aspilia africana absorbed higher concentrations of Zn from the soil compared to other metals, while Chromolaena odorata absorbed more of the other metals(Pb, Cd, Ni, and Cr)than Zn in the rainy season. This is similar to the observations of Olayiwola(2013) . In the dry season collection, the concentration of Zn was higher in Chromolaena odorata in the polluted site, but in the control site no difference was observed(Figures 7-8). The results showed that the concentration of Zn in both Aspilia africana and Chromolaena odorata was higher than the concentration of other elements. Zn usually occurs in low concentrations and does not pose a toxicity problem for plants, but high concentrations of Zn in soil can lead to toxic effects in plants(Chaney and Oliver, 1996). However, the results obtained from this study suggest that different the plant species present on the site had varying abilities to take up and accumulate metals.
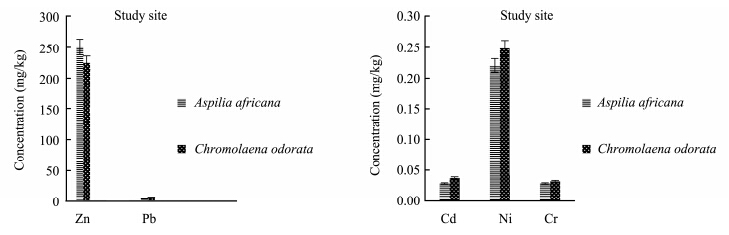 |
| Figure 5 Zn, Pb, Cd, Ni, and Cr concentrations in both Aspilia africana and Chromolaena odorata around the iron smelting factory in the rainy season |
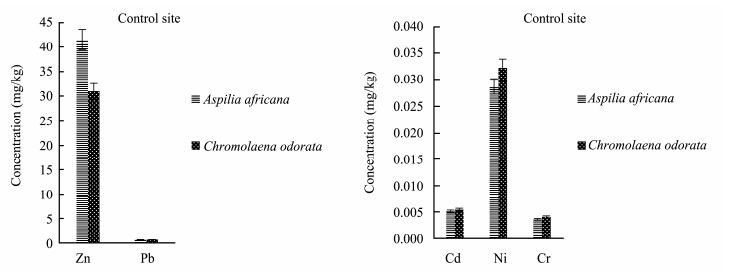 |
| Figure 6 Zn, Pb, Cd, Ni, and Cr concentrations in both Aspilia africana and Chromolaena odorata at the control site in the rainy season |
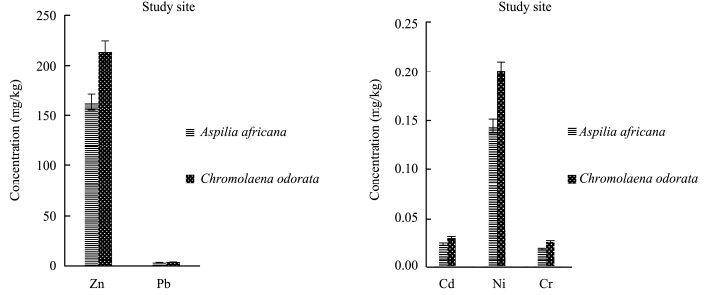 |
| Figure 7 Zn, Pb, Cd, Ni, and Cr concentrations in both Aspilia africana and Chromolaena odorata around the iron smelting factory in the dry season |
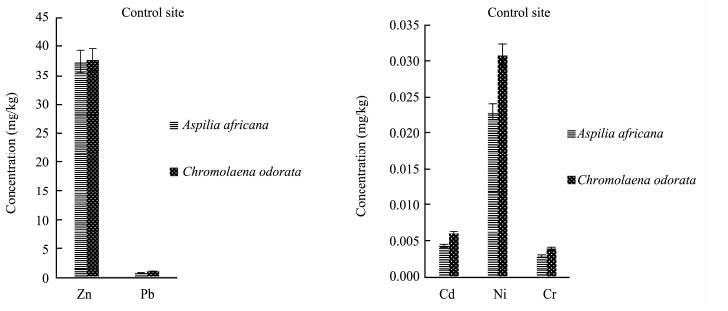 |
| Figure 8 Zn, Pb, Cd, Ni, and Cr concentrations in both Aspilia africana and Chromolaena odorata at the control site in the dry season |
Evaluation of soil and plant contamination status in and near industrial areas is an important environmental issue. This study revealed that near an iron smelting factory located at the Fashina Area, Ile-Ife, Osun State, southwestern Nigeria, heavy metal concentration varied with the local plant species and also with the prevailing seasonal conditions. The accumulation and concentration of heavy metals in two studied plant species and their soil indicated a potential hazard of the factory to the environment, although the levels were lower than the known toxic thresholds. It is therefore advisable that future steel-making facilities should be sited far away from residential and agriculture areas. The data obtained from this research work can be used as baseline data for assessing the impact of this particular factory on the environment because it just began operation.
| Babich H, Schiffenbauer M, Stotzky G, 1982. Comparative toxicity of trivalent and hexavalent chromium to fungi. Bulletin of Environmental Contamination and Toxicology, 28: 193–202. |
| Chaney RL, Oliver DP, 1996. Contaminants and the Soil Envi-ronment in the Australia-Pacific Region. Kluwer Academic Publishers, Dordrecht, pp. 259. |
| Chunilall V, Kindness A, Jonnalagadda SB, 2005. Heavy metal uptake by two edible Amaranthus herbs grown on soils contaminated with lead, mercury, cadmium and nickel. Journal of Environmental Science and Health, 40: 375–384. |
| Cvetanovska L, Kratovalieva S, Girazova E, 2005. Anatomic and physiological disorder after intoxication with heavy metals in tobacco (Nicotiana Tabacum L.). Natura Montenegrina. Pod-gorica, 4: 99–103. |
| El-Demerdash FM, Elgamy EJ, 1999. Biological effects in Tilapia nitotica Fish as indicator of pollution by cadmium and mercury. International Journal of Environmental Health Research, 9: 173. |
| Filazi A, Baskaya R, Kum C, et al., 2003. Metal concentration in tissues of the Black sea fish Mugil auratus from Sinop-Iclimari, Turkey. Human and Experimental Toxicology, 22: 85–87. |
| Gune A, Alpasalan M, Inal A, 2004. Plant growth and fertilizer. Publication No. 1539, Ankara University of Agriculture, An-kara, Turkey. |
| Ilechukwu I, Osuji LC, 2013. Physico-chemical characteristics of soils within the vicinity of a hot mix asphalt (HMA) plant in Obigbo, Port Harcourt, Nigeria. Applied Science Research, 5(3): 184–192. |
| Kerbir T, Bouhadjera K, 2011. Effect of heavy metals pollution in soil and plant in the industrial area west Algeria. Journal of Korean Chemical Society, 55(6): 1018–1028. |
| Nadal M, Schuhmacher M, Domingo JL, 2004. Metal pollution of soils and vegetation in a petrochemical industry. Science of Total Environment, 321: 59–69. |
| Nayak R, Sett R, Biswal D, 2012. Variation in sensitivity of two economically important plants to thermal power plant emis-sions, Angul District, Orissa, India. International Research Journal of Environment Sciences, 1(3): 17–26. |
| Olayiwola OA, 2013. Accumulation and contamination of heavy metals in soil and vegetation from industrial area of Ikirun, Osun State, Nigeria. Global Journal of Pure and Applied Che-mistry Research, 1(1): 25–34. |
| Onder S, Dursun S, Gezgin S, et al., 2007. Determination of heavy metal pollution in grass and soil of city centre green areas (Konya, Turkey). Polish Journal of Environmental Studies, 16(1): 145–154. |
| Sterritt RM, Lester JN, 1980. Interactions of heavy metals with bacteria. Science Total Environment, 14: 5–17 . |
| Stewart E, Allen H, Max GW, et al., 1974. Chemical Analysis of Ecological Materials. Blackwell Scientific Publications, Oxford, UK. |
| Yahaya MI, Mohammad S, Abdullahi BK, 2009. Seasonal varia-tions of heavy metals concentration in abattoir dumping site soil in Nigeria. Journal of Applied Sciences and Environmental Management, 13(4): 9–13. |
 2015, 7
2015, 7


